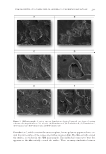
J. Cosmet. Sci., 69, 165–173 (May/June 2018) 165 Preformulation, Characterization, and In Vitro Release Studies of Caffeine-Loaded Solid Lipid Nanoparticles DERYA ALGUL, GULENGUL DUMAN, SAMET OZDEMIR, EBRU TURKOZ ACAR, and GULGUN YENER, Department of Pharmaceutical Technology, Faculty of Pharmacy, Yeditepe University, Istanbul 34755, Turkey (D.A., G.D., S.O.), Department of Pharmaceutical Analytical Chemistry, Faculty of Pharmacy, Yeditepe University, Istanbul 34755, Turkey (E.T.A.), and Department of Pharmaceutical Technology, Faculty of Pharmacy, Istanbul University, Istanbul 34116, Turkey (G.Y.) Accepted for publication January 28, 2018. Synopsis Encapsulation of active agents in solid lipid nanoparticles (SLNs) is an alternative to other controlled release systems for topical delivery. In this study, caffeine was encapsulated in SLNs to produce a delivery system with controlled release. Caffeine-loaded SLNs (Caf-SLNs) were prepared using the double emulsion method with homogenization and ultrasonication. The characterization studies were performed using dynamic light scattering (DLS), zeta potential, scanning electron microscopy (SEM), and differential scanning calorimetry (DSC) analyses. The encapsulation effi ciency tests were performed using UV spectrophotometry. In vitro release studies were conducted using a dialysis bag technique and high-performance liquid chromatography (HPLC) for the quantifi cation of caffeine (Caf). The results from the DLS analysis showed that all formulations had a polydispersity index 0.3 with particle sizes 210 nm. The DSC and SEM results showed that Caf was dispersed in the SLNs. The encapsulation effi ciency was 49.22%. The release studies indicated that after an initial burst at 3 min, the SLNs released Caf in a controlled manner over a 6-h period. Taken together, the SLNs can be used as a carrier for the topical delivery of Caf. INTRODUCTION Delivering active agents across the stratum corneum is diffi cult because of their physico- chemical structures such as lipophilicity and molecular weight. Thus, scientists have focused on topical delivery systems to carry active agents with hydrophilic properties (partition coefficient values or LogP between one and four) and high molecular weights ( 500 g/mol) (1,2). Caffeine (Caf) is a water-soluble active agent with low molecular weight (194.2 g/mol) and favorable LogP value (-0.07) (1). It is topically used for reduc- tion of cellulite appearance and UVB-induced skin cancer treatment (3,4). Several groups have used colloidal particulate carrier systems for the topical delivery of Caf (5–7). Solid Address all correspondence to D. Algul at deryaalgul@gmail.com.
JOURNAL OF COSMETIC SCIENCE 166 lipid nanoparticles (SLNs), a colloidal delivery system, consist of a high melting point solid lipid forming a lipid matrix surrounded with surfactant (5,8). SLNs have several advantages including high tolerability, rapid biodegradation, high bioavailability, high drug-loading capacity, good production scalability, and a lack of organic solvents in the preparation process. SLNs can also provide a controlled release profi le for many active agents and increase the skin penetration rates of the active agents (3,8). Homogenization, solvent emulsifi cation or evaporation, ultrasonication, and solvent diffusion methods are used to prepare SLNs (9). The aim of the present study was to prepare SLNs containing Caf with controlled release and to evaluate the formulations with in vitro experiments. MATERIALS AND METHODS MATERIALS Softisan 100® (Hydrogenated Coco-Glycerides) was obtained from Cremer Oleo GmbH & Co. KG (Hamburg, Germany). Tween 20® (Polysorbate 20), Span 20® (Sorbitan Laurate), and Caf were purchased from Merck KGaA (Darmstadt, Germany). All organic solvents and other chemicals were analytical grade and obtained from Merck KGaA. PREPARATION METHOD FOR CAF-SLNS Caf was dissolved in 80°C water. The aqueous solution was added into the oil phase which comprised a Softisan 100® and Span 20® mixture at the same temperature. It was mixed under constant stirring (27,000 rpm) with a Silent Crusher M homogenizer (Heidolph®, Schwabach Germany) at 80°C for 2 m to obtain a primary emulsion (w/o). Finally, Tween 20® dissolved in hot water (80°C) was slowly added into the emulsion with constant stir- ring (27,000 rpm) at 80°C for 10 m to produce a double emulsion (w/o/w). To decrease the particle size, the emulsion was sonicated using an Bandelin SonoPlus HD 2070 probe type sonicator (Bandelin®, Berlin, Germany) at 70% amplitude level for 2 min to obtain a nanoemulsion (6). FREEZE-DRYING OF SLN DISPERSIONS The nanoemulsions were frozen at -20°C for 5 h and lyophilized in a Alpha 2-4 LSCplus freeze dryer (Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) for 48 h. The SLNs were collected for use in differential scanning calorimetry (DSC) analyses (8). PARTICLE SIZE AND ZETA POTENTIAL MEASUREMENTS The mean diameter, polydispersity index (PI), and zeta potential of each sample were obtained using a Malvern Zetasizer Nano ZS (Malvern Instruments, U.K.) at 25°C. Before all measurements, SLNs were diluted with distilled water (10).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)