FILM PROPERTIES OF POLYMERS USED IN ANHYDROUS SUNSCREEN FORMULATIONS 205 FORMULATION PROCEDURE In all formulations, phases A and B were weighed separately in different beakers. Phase B was heated to 50°C until all crystals were dissolved and then brought back to room tem- perature. Phase A was added to phase B and then ingredients of phase C were added to their respective formulations. Formulations were fi lled into an aluminum can and aero- solized with 30% isobutane (A-31). The actuator was a Moritz twist-to-lock with a Misty 0.025 insert from Aptar (Crystal Lake, IL). VAPOR FLUX METHODOLOGY Evaporimeter measurements were performed using an AquaFlux Model AF200 (Biox Systems, Ltd., London, UK) on pig skin that was placed in a Franz diffusion cell assem- bly. The evaporimeter was fi tted with an adapter to fi t directly onto the Franz diffusion cells. Dermatomed pig skin was cut into 400 mm2 sections and placed over the donor chamber. Test samples were applied on the pig skin in quantities of 2 mg/cm2 using an analytical balance and then spread evenly with a fi nger cot. The receptor fl uid was fi lled with deionized water and the temperature of the skin and the receptor fl uid was kept constant at 34°C using a circulating water bath. Skin was equilibrated for 15 min at that temperature before sample application. Data collection (1 point/s) took place over a period of 60 min to monitor the variations in vapor transport as a function of time. TAPE STRIPPING PROTOCOL Products were sprayed onto a stratum corneum layer that was formed on a D-Squame disc. Standard 22 mm D-Squame discs (CuDerm Corporation, Dallas, TX) were used to Table I Fo rmulations Tested Ingredients Formulations A B C D E F Phase A Alcohol, SD 40-B (200 proof) 93.00 94.00 54.00 52.00 51.00 51.80 Butylene glycol 2.00 2.00 2.00 2.00 2.00 2.00 Phase B Butyl methoxydibenzoylmethane 3.00 3.00 3.00 3.00 Benzophenone-3 6.00 6.00 6.00 6.00 Homosalate 15.00 15.00 15.00 15.00 Ethylhexyl salicylate 5.00 5.00 5.00 5.00 Octocrylene 10.00 10.00 10.00 10.00 Isostearyl neopentanoate 5.00 5.00 5.00 5.00 5.00 Phase C VA/butyl maleate/isobornyl acrylate copolymer (and) alcohol (50% w/w solution in alcohol) 4.00 2.00 2.00 2.00 Hydroxypropyl cellulose 0.20 Acrylates/dimethicone copolymer (and) dimethicone (40/60% w/w) 1.00 Total 100.00 100.00 100.00 100.00 100.00 100.00
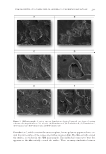
JOURNAL OF COSMETIC SCIENCE 206 collect stratum corneum from the volar forearm of volunteers. Products were sprayed onto the isolated corneocytes from an aerosol placed about 12 inches away from the stratum corneum. The products were left to air-dry for a minimum of 20 min before coating with conductive metals and placing into the scanning electron microscopy (SEM) chamber. SEM PROTOCOL Treated D-Squame discs were placed on 25 mm OD Pelco Tabs (Ted Pella, Inc., Redding, CA). The pin stubs were placed in a sputter coater (Leica EM ACE600 Leica Microsys- tems, Wetzler, Germany) and coated for 60 s with gold/palladium, resulting in a 10-nm thick layer. The stubs were attached to a multisample mount and placed into the SEM (Hitachi SU-5000, Tokyo, Japan), which has variable pressure and fi eld emission scan- ning capabilities. For the analysis of the coated stratum corneum layers, we used high vacuum mode ( 1 × 10-3 Pa) and collected photomicrographs starting at ×300 and extending to higher magnifi cations to elucidate details associated with the sunscreen ap- plication. Most images were collected using a secondary electron detector, but a backscatter detector was also used for samples that exhibited poor contrast properties. 3D images were also captured by SEM. RESULTS In this study, we sought to determine key properties of sunscreen preparations applied to skin. Using evaporimetry in conjunction with a Franz diffusion cell apparatus and ex vivo skin, vapor fl ux data were generated for the sunscreens, allowing for the determination of the water permeability of the sunscreen fi lms. Employing SEM, we developed a novel methodology for determining the morphological fi lm properties when sunscreens are ap- plied to skin. The utility of the technique, aimed at discerning the microscopic fi lm properties, lies in the use of layers of corneocyte cells collected by tape stripping. In this manner, the substrate has the same surface properties as in vivo skin. EVAPORIMETER STUDIES Four formulations were tested in this study, namely, Formulations C, D, E, and F. Because Formulations A and B consist mostly of alcohol, their water permeability was not tested. Instead, water and sunfl ower seed oil were used as negative and positive controls and yielded a cumulative evaporation of 88.0 × 103 and 12.6 × 103 g m-2 h-1, respectively. Both the positive and negative controls were signifi cantly different from all treatments and from each other. The data generated for the four formulations studied are displayed in Figure 1. Formulations C, D, E, and F yielded cumulative evaporations of 53.4, 37.7, 46.0, and 36.6 × 103 g m-2 h-1, respectively. Only Formulation C was signifi cantly differ- ent (when comparing Formulations C, D, E, and F), in which case there was a greater rate of evaporation. Formulations D, E, and F, which contained VA/butyl maleate/isobornyl acrylate copolymer and its combination with acrylates/dimethicone copolymer or hydroxypropyl cellulose,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)