JOURNAL OF COSMETIC SCIENCE 398 To fi ght the global water crisis, we see more and more water conservation efforts being under- taken by consumers, including cutting down on the use of water in personal grooming practices. Consumer awareness of this issue is on the rise, and water conservation is becom- ing a top priority for them. As consumers are cutting back on their water usage, they are expecting personal care brands to do the same (3). Sustainability is becoming more and more integral to the business model of consumer goods companies as it is becoming increas- ingly important in the minds of consumers. Essentially, consumers need products that require less rinsing without compromising the performance of the product. In an effort to reduce the water footprint in the personal care industry, a few companies have started to develop water-smart products with a primary focus on “faster rinsing” claims with the aim of conserving water. However, there is currently no method available to describe the rinsing behavior of products. To live a life of responsible consumption and production, a method is needed to quantify the rinsability of products to prove that they do, in fact, rinse out faster than your conventional shower product. Rinsability is a term coined by the industry to describe the rinsing behavior of personal care products. It is not a new concept. Many companies have developed their own rinsability method by focusing on hair attributes such as wet friction or bending force. However, these methods aim to only focus on the hair itself. Although this is a good starting point, they fail to analyze the product as it washes off the hair. For this reason, studies of the rinsed water have been conducted, using conditioner systems, to describe the rinsing per- formance of such systems and to approximate what ultimately remains on the hair surface. MATERIALS AND METHODS Although conditioners are generally composed of 80–90% water, the remaining percent- age is composed of miscellaneous ingredients that are insoluble in water, thus creating a colloidal system. We can take advantage of the colloidal system to approximate the con- centration of product that is washed off using properties of light scattering. Light scattering is simply the redirection of light it happens when energy waves are forced to deviate from a straight path because of imperfections in a given medium (4). Light scatter can be used to approximate unknown amounts of conditioner material in water by the Beer–Lambert law. The Beer–Lambert law, or A = εmCℓ, is a linear relationship between absorbance and concentration. It is normally used for samples that absorb light at a particular wave- length. However, because conditioner materials mainly scatter light, optical density is used instead of absorbance. Optical density is not a measure of absorbance, but rather a measure of light scattered by particle suspension which manifests itself as absorbance. As visible light passes through a suspension, the light is scattered. Greater scatter indicates more particles present. This method is often used in biological related fi elds to study bacteria that are often colorless and do not scatter light (5). The optical density is represented in terms of transmittance. Transmittance is inversely proportional to absorbance, given by the Beer– Lambert Law. Traditionally, spectrophotometers are used to measure absorbance. However, they are not optimized for light scattering measurements. Spectrophotometers commonly result in differences in measurements between scans and between instruments (6). Because of the nature of the samples, the Turbiscan from Formulaction was used for this study. The Turbiscan uses multiple light scattering theory associated to a vertical scanning head, enabling it to measure particles of varying size and movement. The Turbiscan is used here to obtain transmittance values for our samples (7).
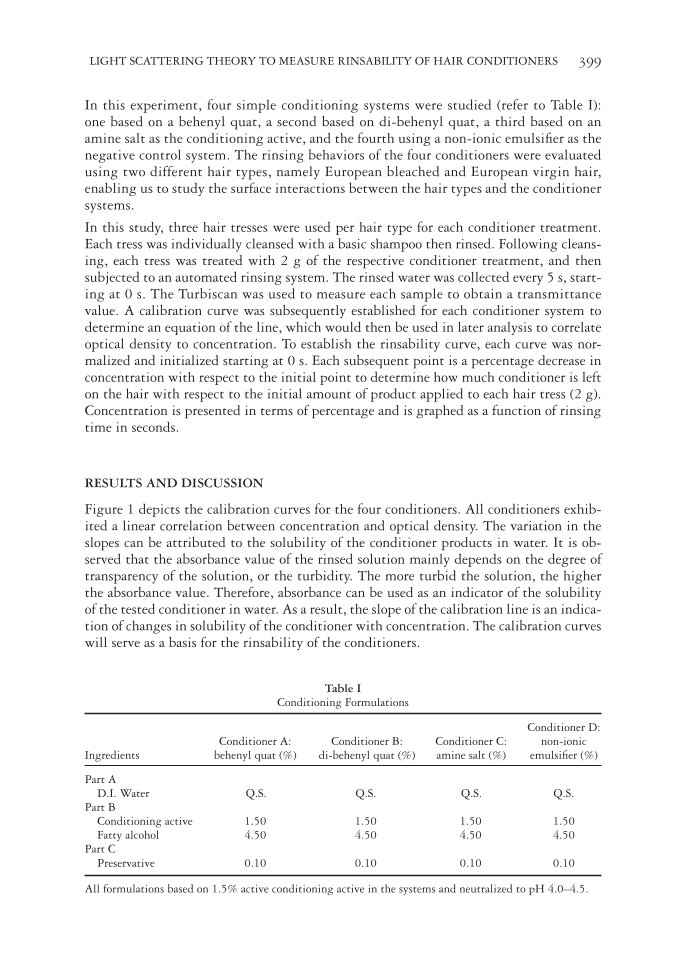
LIGHT SCATTERING THEORY TO MEASURE RINSABILITY OF HAIR CONDITIONERS 399 In this experiment, four simple conditioning systems were studied (refer to Table I): one based on a behenyl quat, a second based on di-behenyl quat, a third based on an amine salt as the conditioning active, and the fourth using a non-ionic emulsifi er as the negative control system. The rinsing behaviors of the four conditioners were evaluated using two different hair types, namely European bleached and European virgin hair, enabling us to study the surface interactions between the hair types and the conditioner systems. In this study, three hair tresses were used per hair type for each conditioner treatment. Each tress was individually cleansed with a basic shampoo then rinsed. Following cleans- ing, each tress was treated with 2 g of the respective conditioner treatment, and then subjected to an automated rinsing system. The rinsed water was collected every 5 s, start- ing at 0 s. The Turbiscan was used to measure each sample to obtain a transmittance value. A calibration curve was subsequently established for each conditioner system to determine an equation of the line, which would then be used in later analysis to correlate optical density to concentration. To establish the rinsability curve, each curve was nor- malized and initialized starting at 0 s. Each subsequent point is a percentage decrease in concentration with respect to the initial point to determine how much conditioner is left on the hair with respect to the initial amount of product applied to each hair tress (2 g). Concentration is presented in terms of percentage and is graphed as a function of rinsing time in seconds. RESULTS AND DISCUSSION Figure 1 depicts the calibration curves for the four conditioners. All conditioners exhib- ited a linear correlation between concentration and optical density. The variation in the slopes can be attributed to the solubility of the conditioner products in water. It is ob- served that the absorbance value of the rinsed solution mainly depends on the degree of transparency of the solution, or the turbidity. The more turbid the solution, the higher the absorbance value. Therefore, absorbance can be used as an indicator of the solubility of the tested conditioner in water. As a result, the slope of the calibration line is an indica- tion of changes in solubility of the conditioner with concentration. The calibration curves will serve as a basis for the rinsability of the conditioners. Table I Con ditioning Formulations Ingredients Conditioner A: behenyl quat (%) Conditioner B: di-behenyl quat (%) Conditioner C: amine salt (%) Conditioner D: non-ionic emulsifi er (%) Part A D.I. Water Q.S. Q.S. Q.S. Q.S. Part B Conditioning active 1.50 1.50 1.50 1.50 Fatty alcohol 4.50 4.50 4.50 4.50 Part C Preservative 0.10 0.10 0.10 0.10 All formulations based on 1.5% active conditioning active in the systems and neutralized to pH 4.0–4.5.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)