ELECTROPHORETIC MOBILITY OF SOME TATTOO DYES 293 NaCl containing no dissolved dyes). Finally, the balancing faucet (Figure 2, 4) was opened to equalize the coupling fl uid levels in both parts of the tube. Aft erward, both extremities of the U-shaped tube were connected electrically to the power supply through additional agar–agar bridges installed between each bend of the tube and the power supply outputs. Then the main faucets (2) were opened and voltage was applied between both parts of the glass tube. Bec ause the dye grains acquire some electric charge in the isotonic solution, they will move toward one of the poles causing a level difference between working and coupling liq- uids. Having measured the difference between the margins in each bend, the zeta poten- tial can be calculated by the following formula (11): ζ = ηhl Eεε 0 t, w her e η is the viscosity of working solution (because all working solutions were quite diluted, it was taken as equal to the viscosity of water 1 × 10-3 Pa s) h is the fi nal differ- ence between the solution levels in each bend, m l is the distance between the axes of two parts of the U-shaped tube (0.58 m) E is the electric voltage applied (260 V throughout all experiments) ε 0 is the standard vacuum dielectric permittivity constant (8.85 × 10-12 F/m) ε is the relative dielectric permissivity of water (equal to 81) and t is the duration of electrophoresis (s). Figure 1. Samples of tattooing pigments used for investigation of the electrophoretic activity.
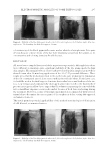
JOURNAL OF COSMETIC SCIENCE 294 Figure 2. U-shaped tube for experimental determination of zeta potential. A difference in the liquid levels was achieved as a result of electrophoresis. (1) Working part fi lled with the dye solution (2) main faucets (3) coupling fl uid parts and (4) balancing faucet.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)