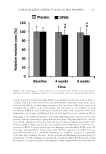
DEVELOPMENT OF ANTIOXIDANT SKIN GEL USING COFFEE SILVERSKIN 315 MATE RIALS AND METHODS MATE RIALS Robu sta coffee silverskin was obtained from a coffee roasting company located in Bandar Lampung, Indonesia. Technical grade ethanol (96% v/v) was purchased from PT Sumber Abadi (Tangerang, Indonesia). Sodium carbonate (BDH, London, England), Folin– Ciocalteu reagent (Merck, Darmstadt, Germany), gallic acid (GA) powder (Aktin Chemical, Chengdu, China), aluminum chloride (Merck), potassium acetate (BDH), 2,2-diphenyl- 1-picrylhydrazyl (DPPH, Sigma-Aldrich, St. Louis, MO), and analytical grade ethanol (Smart Lab, Bogor, Indonesia) were used. Chemicals to prepare the basic skin gel were carbomer 940, propylene glycol, methylparaben, and propyl paraben, all purchased from PT Intralab Ekatama (Bogor, Indonesia), whereas triethanolamine (TEA) and ethylene diamine tetra acetic acid (EDTA) were purchased from PT Sumber Abadi. Distilled water was used. EQUI PMENT Stan dard laboratory equipment was used in this work, namely, hot plate (Cimarec, Waltham, MA), beaker glasses, Erlenmeyer fl asks, magnetic stirrer, thermometer, volumetric glass (Pyrex, Corning, NY), water bath shaker (Memmert, Schwabach, Germany), micropipette (Eppendorf, Hamburg, Germany), micropipette tips, cuvette (Brand GMBH, Wertheim, Germany), rotary vacuum evaporator (IKA HB 10, Shanghai, China), spray dryer (BUCHI mini spray dryer B-290, Flawil, Switzerland), analytical balance (Ohaus PA214, Parsip- pany, NJ), UV-Vis spectrophotometer (Genesys 10-S, Waltham, MA), vortex (Vortex- Genie 2, St. Louis, MO), fi lter instrument, Whatmann fi lter paper 1001 125 (GE, Little Chalfont, Buckinghamshire, UK), desiccator, moisture content analyzer (Sartorius MA35, Goettingen, Germany), mixer (IKA Labortechnik, Staufen, Germany), pH meter (Lutron pH-208, Taipei, Taiwan), and viscometer (Brookfi eld DV-E, Lorch, Germany). EXTR ACTION PROCEDURE Firs t, the coffee silverskin sample was rinsed with water and dried in an oven at 40°C. The dried sample was milled using a mixer grinder to reduce the size. Then, 100 mL of water– ethanol mixture with a weight ratio of 50:50 was poured into a glass beaker and heated using a hot plate. The temperature was varied at 30, 40, 50, and 60°C. When the desired tem- perature of the solvent was reached, 2 g of coffee silverskin were poured into the solvent, and the extraction process was carried out by stirring using a magnetic stirrer at a speed of 350 rpm. Thus, the weight ratio of the coffee silverskin and water–ethanol solvent was 1:50. The beaker was covered with aluminum foil to prevent loss of solvent because of evapora- tion during heating. The extraction time was varied at 5, 10, 20, 30, 40, and 60 min. The extract solution was then fi ltered using a fi lter paper and stored in a refrigerator before analysis. ANALYS IS OF TOTAL PHENOLIC CONTENT AND ANTIOXIDANT ACTIVITY The to tal phenolic content was determined by using the Folin–Ciocalteu method. First, the F olin–Ciocalteu reagent solution was made by diluting concentrated Folin–Ciocalteu
JOURNAL OF COSMETIC SCIENCE 316 reagent in distilled water at a ratio of 1:10. Sodium carbonate solution (7.5% w/v) was made by diluting 7.5 g of solid sodium carbonate with 100 mL of distilled water. For the standard curve of GA, a solution of GA was prepared by dissolving 0.1 g of solid GA in 100 mL of distilled water to obtain 1,000 mg/L of GA stock solution. Then the standard solutions of GA were made by diluting the GA solution into concentrations of 10, 20, 50, 70, 100, 250, and 500 mg/L. Furthe rmore, the coffee silverskin extract solution was diluted with a weight ratio of 1:10, and 500 μL of the diluted extract solution was mixed with 2.5 mL of the Folin– Ciocalteu reagent solution and 2 mL of the sodium carbonate solution. The mixture was then mixed using a vortex mixer and incubated in the dark at room temperature for 1 h. After the incubation, the mixture was poured into a cuvette and directly checked by using a UV-Vis spectrophotometer at 765 nm to measure the absorbance. The total phenol content (TPC) was then calculated by using the following equation: qDF Abs Total Phenolic Content (mg GAE/L) , m (1) wh ere Ab s is the absorbance, m is the gradient of the GA standard curve, and DF is the dilution factor. The total phenolic content was expressed in mg of GA equivalent per liter extract solution [mg Gallic Acid Equivalent (GAE)/L] and then converted to mg GAE/g dry coffee silverskin (mg GAE/g CS). To deter mine the antioxidant activity of the extract, the extract samples were tested using the DPPH free radical scavenging assay. A stock solution of DPPH (250 μM) was pre- pared by diluting 11 mg of DPPH powder in 20 mL ethanol (96% v/v). The stock solu- tion was covered with aluminum foil and stored at a temperature of 4°C. Next, 100 μL DPPH, 50 μL sample, and 850 μL ethanol were mixed in a test tube. For the control, 100 μL DPPH, 50 μL distilled water, and 850 μL ethanol were mixed in a test tube. Further- more, both test tubes were wrapped with aluminum foil and stored in a dark room for 30 min. The absorbance reading was performed using a UV-Vis spectrophotometer at a wavelength of 515 nm. The antioxidant activity was expressed as an inhibition percent- age and calculated by using the following equation: q100%, ( ) Antioxidant Activity % c s c A A A (2) where Ac is th e a bsorbance of the control and As is the absorbance of the sample. The antioxidant activity (the free radical scavenging activity) obtained by this method was expressed as IC50 (in ppm), which means the concentration of the sample needed to in- hibit 50% of the DPPH as the free radical. STATISTICAL ANALY SIS Statistical analy sis of the data obtained in this work was performed by using analysis of variance and Tukey test. The difference among data of different samples was considered as signifi cant if the probability was less than 0.05 (p 0.05).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)