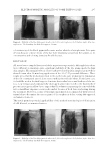
ELECTROPHORETIC MOBILITY OF SOME TATTOO DYES 297 color intensity of the black pigment becomes weaker after the electrophoresis. It is quite obvious because some portions of the dye were migrating away from the sample, so its concentration and coloring intensity should decrease. DISCUSSION Al l tested ta ttoo samples have revealed the negative zeta potentials, although their values were suffi cient to maintain quite signifi cant mobility of the dye grains inside the faux skin samples. The margin between colored and not-colored gelatin samples has moved for about 10 mm after 40-min-long application of the 20–25 V potential difference. These results prove that the method presented in this work looks quite promising for elimination of the old or unwanted tattoos alone or in combination with the others. In the latter case, it should be used at the fi nal stage to eliminate the residual traces and shadows of the tat- toos. Low voltages and comparatively short application time tested in the framework of present investigation allow one to expect that the volunteer requesting the removal of the tattoo should not experience serious discomfort because of local skin overheating during the treatment. However, a series of thorough experiments is recommended with tattooed animal skin to determine the exact regimes of electrophoresis before testing this approach on human volunteers. This result p romises potential applicability of this method in technologies of elimination of old, blurred, or unwanted tattoos. Figure 4. Mobility o f the black dye particles after the 2,400-s electrophoresis. Left, before right, after elec- trophoresis. The boundary has shifted for approx. 10 mm. Figure 5. Mobility of the black dye particles after the 2,400-s electrophoresis. Left, before right, after elec- trophoresis. The boundary has shifted for approx. 8 mm.
JOURNAL OF COSMETIC SCIENCE 298 REFERENCES ( 1) K. Burri s and K. Kim, Tattoo removal, Clin. Dermatol., 25(4), 388–392 (2007). (2) L. I. Nag a and T. S. Alster, Laser tattoo removal: an update, Am. J. Clin. Dermatol., 18(1), 59–65 (2017). (3) S. G. Ho and C. L. Goh, Laser tattoo removal: a clinical update, J. Cutan. Aesthet. Surg., 8(1), 9–15 (2015). (4) N. Khung e r, A. Molpariya, and A. Khunger, Complications of tattoos and tattoo removal: stop and think before you ink, J. Cutan. Aesthet. Surg., 8(1), 30–36 (2015). (5) N. Kluge r , S. Hakimi, and P. Del Giudice, Keloid occurring in a tattoo after laser hair removal, Acta Derm. Venereol., 89(3), 334–335 (2009). (6) P. D. Gr a ham, P. T. Elliott, and K. G. Gallagher, United States Patent 7179253B2, accessed February 20, 2019, https://patentimages.storage.googleapis.com/a6/49/ec/51058b4edf0876/US7179253.pdf. (7) L. Song, Z. Ning, and T. Min, The wettability with Ca2+ brine in a tight oil reservoir with zeta poten- tial, Pet. Sci. Technol., 36(24), 2106–2111 (2018). (8) A. M. Ja s trzebska, J. Karcz, R. Letmanowski, D. Zabost, E. Ciecierska, J. Zdunek, E. Karwowska, M. Siekierski, A. Olszyna, and A. Kunicki, Synthesis of the RGO/Al2O3 core-shell nanocomposite fl akes and characterization of their unique electrostatic properties using zeta potential measurements, Appl. Surf. Sci., 362, 577–594 (2016). (9) Z. Wu, J. Wu, R. Zhang, S. Yuan, Q. Lu, and Y. Yu, Colloid properties of hydrophobic modifi ed algi- nate: surface tension, ζ-potential, viscosity and emulsifi cation, Carbohydr. Polym., 181, 56–62 (2018). (10) H. J. Jac o basch, G. Baubock, and J. Schurz, Problems and results of zeta-potential measurements on fi bers, Colloid Polym. Sci., 263, 3–24 (1985). (11) J. T. G. Overbeek and P. H. Wiersema, “The Interpretation of Electrophoretic Mobilities,” in Electropho- resis: Theory, Methods and Applications. M. Bier. Ed. (Academic Press. London, New York, 1959), Vol. 1, pp. 1–52. (12) A. Strickle r and T. Sacks, Focusing in continuous-fl ow electrophoresis systems by electrical control of effective cell wall zeta potentials, Ann. N.Y. Acad. Sci., 209, 497–514 (2006). (13) D. Michaud, L. Cantin, D. A. Raworth, and T. C. Vrain, Assessing the stability of cystatin/cysteine proteinase complexes using mildly-denaturing gelatin-polyacrylamide gel electrophoresis, Electrophoresis, 17(1), 74–79 (1996).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)