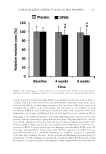
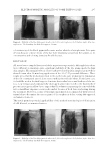
PREFORMULATION STUDIES OF PEPTIDES KTTKS AND PAL-KTTKS 311 represent crystal habits as well. In this regard, some habits such as needle-likes might provide some degrees of discomfort in topical applications and should be considered in the formulation and stability studies by the formulator. CONCLUSION Here, KTTKS and P al-KTTKS were synthesized and the physicochemical properties of both peptides and, therefore, the effects of covalent attachment of palmitic acid on KTTKS properties were investigated. In short, these peptides showed birefringence, irregular morphology, wide particle size distribution, and no melting point before decomposition. In addition, KTTKS was very hydrophilic in nature, and its aqueous solution remains stable at 32°C for over 48 h. In terms of internal structure, Pal-KTTKS appeared more ordered than KTTKS. Micelle formation of Pal-KTTKS in water occurs at low concentra- tions. The results show that fatty acid attachment to KTTKS causes some changes in chemical properties (such as solubility and partition coeffi cient), whereas physical properties (such as morphology, thermal behavior, and birefringence) are not affected so much. These results can be used for formulation of these peptides for topical delivery or any other drug delivery system development. The effects of palmitic acid conjugation on KTTKS prop- erties might be used for the preparation of palmitic acid derivatives on other peptides. ACKNOWLEDGMENTS This paper is a part of PhD thesis of Seyedeh Maryam Mortazavi at the School of Pharmacy, Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran and was fi nancially supported by SBMU. REFERENCES (1) M. Ramos-e-Si l va, L. Celem, S. Ramos-e-Silva, and A. P. Fucci-da-Costa, Anti-aging cosmetics: facts and controversies, Clin. Dermatol., 31, 750–758 (2013). (2) N. H. Abu Sam a h and C. M. Heard, Topically applied KTTKS: a review. Int. J. Cosmet. Sci., 33, 483– 490 (2011). (3) S. Schagen, T o pical peptide treatments with effective anti-aging results, Cosmetics, 4, 16 (2017). (4) F. Gorouhi an d H. I. Maibach, Role of topical peptides in preventing or treating aged skin, Int. J. Cosmet. Sci., 31, 327–345 (2009). (5) K. Katayama, J . Armendariz-Borunda, R. Raghow, A. H. Kang, and J. M. Seyer, A pentapeptide from type I procollagen promotes extracellular matrix production, J. Biol. Chem., 268, 9941–9944 (1993). (6) C. Lu, B. M. K im, D. Lee, M. H. Lee, J. H. Kim, H. B. Pyo, and K. Y. Chai, Synthesis of lipoic acid- peptide conjugates and their effect on collagen and melanogenesis, Eur. J. Med. Chem., 69, 449–454 (2013). (7) L. R. Robinso n , N. C. Fitzgerald, D. G. Doughty, N. C. Dawes, C. A. Berge, and D. L. Bissett, Topical palmitoyl pentapeptide provides improvement in photoaged human facial skin, Int. J. Cosmet. Sci., 27, 155–160 (2005). (8) J. J. Fu, G. G . Hillebrand, P. Raleigh, J. Li, M. J. Marmor, V. Bertucci, P. E. Grimes, S. H. Mandy, M. I. Perez, S. H. Weinkle, and J. R. Kaczvinsky, A randomized, controlled comparative study of the wrinkle reduction benefi ts of a cosmetic niacinamide/peptide/retinyl propionate product regimen vs. a prescription 0.02% tretinoin product regimen, Br. J. Dermatol., 162, 647–654 (2010). (9) G. B. Fields a nd R. L. Noble, Solid phase peptide synthesis utilizing 9-fl uorenylmethoxycarbonyl amino acids, Int. J. Pept. Protein Res., 35, 161–214 (1990).
JOURNAL OF COSMETIC SCIENCE 312 (10) OECD, Test N o . 107: Partition Coeffi cient (n-octanol/water): Shake Flask Method, OECD Publishing, Paris, France (1995). (11) ACD/Labs, Par tition Coeffi cient Calculation with ACD/LogP, Advanced Chemistry Development, Inc., 2019, accessed June 21, 2019, https://www.acdlabs.com/products/percepta/predictors/logp/. (12) I. V. Tetko, J. Gasteiger, R. Todeschini, A. Mauri, D. Livingstone, P. Ertl, V. A. Palyulin, E. V. Radchenko, N. S. Zefi rov, A. S. Makarenko, V. Y. Tanchuk, and V. V. Prokopenko, Virtual computa- tional chemistry laboratory - design and description. J. Comput. Aided Mol. Des., 19, 453–463 (2005). (13) S. Gaisford, “Pharmaceutical preformulation,” in Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 5th Ed., M. E. Aulton and K. M. G. Taylor. Eds. (Churchill Livingstone, London, 2018), pp. 391–392. (14) ACDLabs, ACD / ChemSketch.12.01 edn (Advanced Chemistry Development, Inc., Toronto, 2009). (15) S. Gaisford, “Pharmaceutical preformulation,” in Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, M. Aulton and K. Taylor. Eds. (Churchill Livingstone, London, 2018), pp. 402. (16) J. Dandurand , V. Samouillan, M. H. Lacoste-Ferre, C. Lacabanne, B. Bochicchio, and A. Pepe, Confor- mational and thermal characterization of a synthetic peptidic fragment inspired from human tropoelas- tin: signature of the amyloid fi bers, Pathol. Biol., 62, 100–107 (2014). (17) J. E. Noble a nd M. J. Bailey, Quantitation of protein, Methods Enzymol., 463, 73–95 (2009). (18) A. R. Field, J. Fink, R. Dux, G. Fußmann, U. Wenzel, and U. Schumacher, A spatially scanning vacuum ultraviolet and visible range spectrometer for spectroscopy of tokamak plasmas in ASDEX-Upgrade, Rev. Sci. Instrum., 66, 5433–5441 (1996). (19) B. B. Stephens, R. F. Keeling, and W. J. Paplawsky, Shipboard measurements of atmospheric oxygen using a vacuum-ultraviolet absorption technique, Tellus B Chem. Phys. Meteorol., 55, 857–878 (2011). (20) N. J. Anthis and G. M. Clore, Sequence-specifi c determination of protein and peptide concentrations by absorbance at 205 nm, Protein Sci., 22, 851–858 (2013). (21) X. Zhu, R. Zou, P . Sun, Q. Wang, and J. Wu, A supramolecular peptide polymer from hydrogen-bond and coordination-driven self-assembly, Polym. Chem., 9, 69–76 (2017). (22) F. F. de Sousa, C . E. S. Nogueira, P. T. C. Freire, S. G. C. Moreira, A. M. R. Teixeira, A. S. de Menezes, J. Mendes Filho, and G. D. Saraiva, Conformational change in the C form of palmitic acid investigated by Raman spectroscopy and X-ray diffraction, Spectrochim. Acta, 161, 162–169 (2016). (23) E. Moreno-Calvo, R. Cordobilla, T. Calvet, F. Lahoz, and A. I. Balana, The C form of n-hexadecanoic acid, Acta Crystallogr. C, 62, o129–o31 (2006). (24) S. Namjoshi, I. T oth, J. Blanchfi eld, N. Trotter, R. Mancera, and H. E. Benson, Enhanced transdermal peptide delivery and stability by lipid conjugation: epidermal permeation, stereoselectivity and mecha- nistic insights, Pharm. Res., 31, 3304–3312 (2014). (25) K. Setoh, M. Mur a kami, N. Araki, T. Fujita, A. Yamamoto, and S. Muranishi, Improvement of trans- dermal delivery of tetragastrin by lipophilic modifi cation with fatty acids, J. Pharm. Pharmacol., 47, 808–811 (1995). (26) J. Møss and H. B u ndgaard, Prodrugs of peptides. 7. Transdermal delivery of thyrotropin-releasing hor- mone (TRH) via prodrugs, Int. J. Pharm., 66, 39–45 (1990). (27) Y. L. Choi, E. J. Park, E. Kim, D. H. Na, and Y. H. Shin, Dermal stability and in vitro skin permeation of collagen pentapeptides (KTTKS and palmitoyl-KTTKS), Biomol Ther., 22, 321–327 (2014). (28) I. W. Hamley, Sel f -assembly of amphiphilic peptides, Soft Matter, 7, 4122–4138 (2011). (29) A. Patist, S. S. B hagwat, K. W. Penfi eld, P. Aikens, and D. O. Shah, On the measurement of critical micelle concentrations of pure and technical-grade nonionic surfactants, J. Surfactants Deterg., 3, 53–58 (2000). (30) A. R. Bilia, L. S c alise, M. C. Bergonzi, and F. Vincieri, Effect of surfactants and solutes (glucose and NaCl) on solubility of Kavain--a technical note, AAPS PharmSciTech, 9, 444–448 (2008). (31) ProImmune, Workin g with Peptides. ProImmune Limited, 2012, accessed December 18, 2018. https:// www.proimmune.com/ecommerce/pdf_fi les/ST55.pdf.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)