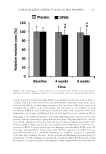
PREFORMULATION STUDIES OF PEPTIDES KTTKS AND PAL-KTTKS 305 STRUCTURE EVALUATION BY XRD XRD was ap p lied to understand the soli d-state form of peptides, which is possibly the most important state in developing a drug candidate into a drug product (15). Patterns of refl ection intensity versus 2θ are shown in Figure 4. As seen, the XRD pattern of palmitic acid displayed an ordered structure. Peaks with the highest intensities are presented in Table I. The XRD pattern of KTTKS shows two peaks in a wide range of Bragg’s angles: one broad peak at 2θ = 19.5 with d-spacing (repeat distance) of 8.7 Å and another at 2θ = 10.1 with d-spacing of 4.5 Å (Figure 4). After peptide modifi cation with palmitic acid (Pal-KTTKS), in addition to two peaks at 2θ = 10.6 (d-spacing = 8.3 Å) and 2θ = 19.1 (d-spacing = 4.6 Å), which are close to that of KTTKS, a peak at 2θ = 21.3 (d-spacing = 4.1 Å) appears. In addition, three other peaks appear in the small angle area: a sharp peak at 2θ = 2.0 with d-spacing of 44.2 Å, a peak at 2θ = 6.5 with d-spacing of 13.6 Å, and a peak at 2θ = 9.5 with d-spacing of 9.34 Å, indicating the presence of a long-range order in Pal-KTTKS. Figure 4. XRD pattern of p a lmitic acid (A) and comparison of XRD patterns of KTTKS and Pal-KTTKS (B) at ambient temperature.
JOURNAL OF COSMETIC SCIENCE 306 PARTITIONING AND SOLUBILITY Partition coeffi cient is a d e termining property which af fects the permeation of a permeant across the skin. The experimental logP value of KTTKS, obtained through the shake fl ask method, was -1.6 ± 0.15. We could not determine logP of Pal-KTTKS experimentally be cause this molecule ap- parently self-aggregates in n-octanol–saturated water even at low concentrations (as low as 25 μg/mL). The same problem occurred when n-hexadecane was used as the oily phase. Lower concentrations were not used because of detection limitations. In such situations, formulators can use different software, such as ACD/ChemSketch, to calculate logP. The calculated logP (clogP) of Pal-KTTKS obtained here by this software was found to be 3.7 (11). Therefore, it could be concluded that the peptide conjugate Pal-KTTKS is much more lipophilic than KTTKS (logP = -1.6) and might be a better candidate for skin permeation than KTTKS. Such a compound might even get trapped in the lipid domain of the stratum corneum. Such a lipophilic molecule is expected to be soluble in lipophilic vehicles, such as ointments or oily phase of o/w and w/o emulsions and creams. KTTKS aqueous solubility was estimated to exceed 220 mg/mL, w hich is considered as freely soluble. However, we were unable to determine the solubility of Pal-KTTKS be- cause of its complex behavior in aqueous systems possibly due to its self-aggregation and assembly. Solubility estimation by ALOGPS revealed an aqueous solubility of 0.02 mM (0.016 mg/mL) for Pal-KTTKS, in good agreement with CMC value obtained by the ring method, as discussed later. SURFACE TENSION MEASUREMENT OF PEPTIDE SOLUTIONS Theoretical and experimental surface tensions of both peptide s as well as deionized water obtained by ACD-ChemSketch software and the ring method, respectively, are pro- vided in Table II. The experimental surface tension of KTTKS aqueous solution was Table II Theoretical and Experimental Surface Tensions of Deionized Water, KTTKS, and Pal-KTTKS Theoretical surface tension (mN/m) Experimental surface tension (mN/m) (n = 3) Deionized water 72.2 ± 3 70.8 ± 0.05 KTTKS 63.1 ± 3 69.0 ± 2.7 Pal-KTTKS 50.1 ± 3 50.3 ± 0.4 Table I Summary of XRD Patterns of Palmitic A cid at Ambient Temperature 2θ (degree) Repeat distance (Å) Relative intensity (%) 2.6 34.6 6.2 7.5 11.7 17.5 20.5 4.3 5.9 21.7 4.1 100 24.3 3.7 18.6 30.3 2.9 4.4
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)