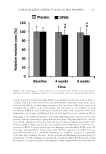
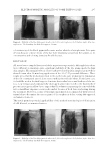
ELECTROPHORETIC MOBILITY OF SOME TATTOO DYES 295 The dye solut ion level did rise in the “positive” bend (Figure 2, right side) for all four samples meaning that the dye grains for all colors used in the framework of our experi- ments acquired a negative charge when dissolved in the isotonic NaCl solution. ANALYSIS OF T HE ELECTROPHORETIC MOBILITY OF PIGMENTS Because the z eta potential of the black pigments was the largest, it was selected for the investigations related to applicability of electrophoresis to extract samples of “real” pig- ments samples from tissues. No human or animal tissue samples were used because of ethical restrictions and gelatin-based mixtures were used at that stage. Twenty-fi ve m illigrams of unbranded gelatin was dissolved in 500 mL of hot water to obtain such mixtures. Then the solution was divided into two parts one of them was left for cooling and hardening, whereas the required amount of the pigment suspension was added to the second one to make its concentration equal to 0.25 mL/L. Then it was also left for cooling and hardening. Parallelepipe dal samples were cut out of each part and placed into a plastic microelectro- phoretic cell (Figure 3). The cell was designed in such a way (12) that each sample was in contact with the graphite electrodes located on the outer edges of the cell and connected to an external electric power supply, whereas the inner sides of the samples were in con- tact with each other ensuring transferability of the dye particles between the samples. Figure 3. Microelec t rophoresis cell with the gelatin samples: (1) raw sample (2) the sample consisting some dye (3) graphite electrodes. Let us compare positions of the boundary between the colored and not-colored samples for various gelatin/black dye samples.
JOURNAL OF COSMETIC SCIENCE 296 Because the d ye particles can move from the colored sample toward the not-colored one under the applied external electric voltage, this process can be visualized by distortion or shifting of the margin between the samples. In case it moves noticeably, the principal applicability of this method to extract the tattoo dyes out of human skin tissues can be expected. All microelec trophoretic experiments involving gelatin samples were carried out under the following conditions: DC voltage applied, 20–25 V current, 5–7 mA and process duration, 2,400 s. It should be noted that such, or close, potential values are used regu- larly in practice at various medical electrophoretic manipulations or biochemical investi- gations (13). RESULTS All e xperimen tal results related to determination of the dyes’ zeta potentials are shown in Table I. As seen from the average zeta potential values given in Table I, the difference between the potentials of various dyes was larger than the experimental error. It means that the dye composition is an infl uential parameter governing the value of its electrokinetic poten- tial. Because the zeta potential of the most popular black pigment was the largest, it should be the most mobile and the most suitable for electrophoretic extraction. There- fore, this pigment was selected for further experiments. As seen from Figures 4 and 5, the black dye’s mobility was quite signifi cant and it reached 10 mm even after the 40-min-long electrophoresis. Besides, it can be noticed that the Table I Experimental D ata and Calculated Zeta Potentials for the Dyes Dye Electrophoresis duration, s Level difference, m ζ, V Red 1,200 0.020 −0.135 0.020 −0.135 0.024 −0.162 Average 0.021 −0.142 ± 0.0119 Black 1,200 0.020 −0.135 0.024 −0.162 0.022 −0.148 0.026 −0.175 0.020 −0.135 Average 0.024 −0.151 ± 0.014 Green 1,200 0.018 −0.121 0.016 −0.108 0.022 −0.148 0.020 0.135 0.026 0.175 Average 0.0204 −0.138 ± 0.0194 White 1,200 0.012 −0.0810 0.016 −0.108 0.02 −0.135 0.02 −0.135 0.014 −0.0944 Average 0.064 −0.111 ± 0.0194
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)