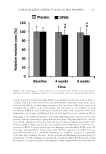
DATE PALM KERNEL EXTRACT ON FACIAL SKIN WRINKLES 281 RESULTS Only 36 subjects of 43 p articipants completed the study (seven male participants, age range 41–55 years, and 29 female participants, age range 40–65 years). Reasons for drop- ping out from follow-up visits were given as holiday time, tiredness, and other family issues rather than any problem with the cream preparations. A preliminary patch test revealed no skin reactions observed at 1, 24, and 96 h after removing the test materials in all 12 subjects. The physicochemical characteristics of both the placebo and test creams, kept at different temperatures for 8 weeks, are shown in Table II. The color was pearly white with no changes in homogeneity or phase separation up to 50 ± 0.1°C and up to 40 ± 0.1°C with 75% relative humidity. The presence of the lipophilic emulsifi er ABIL® EM 90 stabilized the emulsions at high temperatures. CLINICAL ASSESSMENT Clinical assessment of facial skin appearance was conducted at baseline and after comple- tion of the study (i.e., after 8 weeks). Based on a three-point scale evaluation, there were statistically signifi cant improvements in skin roughness, texture homogeneity, and melanin pigmentation. Skin redness levels were not signifi cantly altered in all subjects (Table III). BIOPHYSICAL MEASUREMENTS Meas urements of objective ski n parameters with a multiprobe instrument over 8 weeks revealed progressive increases of surface hydration and elasticity together with a progres- sive decline in both dermal pigmentation and TEWL in DPKE-treated skin compared with placebo cream. Figure 1A illustrates changes of skin hydration at three time points of study. At baseline, the mean values of skin hydration were 41.21 ± 10.32 and 42.10 ± 9.64 AU of placebo and DPKE creams, respectively. After 4 weeks, these values rose to 43.71 ± 11.62 and 52.92 ± 10.25 AU, respectively, and after 8 weeks, the values were 44.21 ± 10.42 and 52.23 ± 11.54, respectively (p 0.01 vs. placebo and p 0.001 vs. baseline for the two time points). Figure 1B illustrates changes of TEWL at three time points of the study. At baseline, the mean readings of TEWL were 12.23 ± 3.11 and 11.76 ± 2.92 g/m2/h of placebo and DPKE creams, respectively. After 4 weeks, these values were 11.72 ± 2.52 and 10.43 ± 2.41 g/m2/h, respectively, and after 8 weeks, the Table II Physicochemical Characteristics of Placebo and DPKE Creams Kept at Different Temperatures for 8 Weeks Color Texture Homogeneity Phase separation Immediate skin feel 5–40 ± 0.1°C White Smooth Homogeneous No Refreshing, cool, no grittiness or greasiness 40 ± 0.1°C with 75% relative humidity White Smooth Homogeneous No Refreshing, no grittiness or greasiness 50 ± 0.1°C White Smooth Slight liquefaction Slight Refreshing, no grittiness or greasiness
JOURNAL OF COSMETIC SCIENCE 282 values were 11.53 ± 2.41 and 10.04 ± 2.73 g/m2/h, respectively (p 0.05 vs. both pla- cebo and baseline at the two time points). Figure 1C and D illustrate changes of ski n elasticity and fi rmness at three time points of the study. At baseline, the mean values of skin elasticity were 0.84 ± 0.08 and 0.84 ± 0.09 AU of placebo and DPKE creams, respectively after 4 weeks, these values were 0.85 ± 0.07 and 0.89 ± 0.06 AU, respectively (p 0.05 vs. placebo and p 0.01 vs. base- line), and after 8 weeks, the values were 0.86 ± 0.09 and 0.94 ± 0.10 AU, respectively (p 0.001 vs. both placebo and baseline). At baseline, the mean fi rmness in the DPKE test skin was 0.27 ± 0.08 AU, and after 4 and 8 weeks, the fi rmness had diminished to 0.23 ± 0.06 and 0.21 ± 0.06 AU, respectively (p 0.01 vs. placebo). Figure 1E illustrates changes of skin melanin density at three time points of the study. At baseline, the mean readings of skin melanin were 273.8 ± 27.9 and 265.6 ± 26.4 AU of placebo and DPKE creams, respectively. After 4 weeks, these values were 267.4 ± 30.2 and 260.8 ± 27.5 AU, respectively, and after 8 weeks, the values were 275.2 ± 28.4 and 255.3 ± 26.5 AU, re- spectively (p 0.05 vs. both placebo and baseline). There were no signifi cant changes in erythema levels for both placebo and DPKE creams over 8 weeks (Figure 1F). ANTERA 3D® MULTISPECTRAL ANALYSIS The signifi cant reduct ion in the wrinkle depth compared w ith pretreatment values was confi rmed by objective analysis with the Antera 3D® analyzer performed at baseline, and after 4 and 8 weeks of treatment. Several wrinkle measurements showed signifi cant im- provements after 4 and 8 weeks compared with both baseline values and placebo cream. With the medium fi lter, there was a signifi cant decline in the overall size after 4 and 8 weeks by 18.4% and 28.6%, respectively (from 56.4 ± 22.2 to 46.0 ± 21.5 (p 0.05 vs. baseline and placebo), and 40.3 ± 22.1 AU (p 0.01 vs. baseline and placebo, paired t-test, Figure 2A). The maximal depth was signifi cantly decreased by 22.5% and 29.6%, respectively (from 0.410 ± 0.19 mm to 0.322 ± 0.18 mm and 0.284 ± 0.23 mm, respec- tively (p 0.05 vs. baseline and placebo for all, paired t-test, Figure 2B). Moreover, sig- nifi cant improvements in the indentation index and skin roughness index were also evident after 4 and 8 weeks however, the reductions of these parameters were more pro- nounced after 8 weeks, compared with baseline and placebo levels (p 0.01 and p 0.001, respectively, Figure 2C and D). The overall reduction in the skin color was also Table III Clinical Assessment Rated on a Three-Point Scale Baseline After 8 weeks Placebo DPKE Skin roughness score 2.24 ± 0.81 2.17 ± 0.64 1.76 ± 0.94**,# Skin texture homogeneity score 2.18 ± 0.76 2.01 ± 0.90 1.55 ± 0.66***,# Skin melanin pigmentation score 2.36 ± 0.95 2.28 ± 0.74 1.90 ± 0.84*,# Skin redness score 2.04 ± 0.84 2.11 ± 0.72 1.93 ± 0.93 Data are presented as mean ± SD of 36 subjects. Signifi cance levels: *p 0.05, **p 0.01, and ***p 0.001 versus baseline. #p 0.05 versus placebo (ANOVA).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)