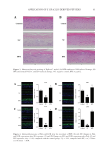
72 JOURNAL OF COSMETIC SCIENCE method in the cosmetics industry improves some basic products’ properties (e.g., efficiency and skin penetration). However, the existing encapsulation techniques may be destructive for the thermosensitive ingredients due to high temperatures needed during encapsulation (24). The electrohydrodynamic process is an inexpensive, nondestructive, and scalable technique used to produce particles (electrospraying) or fibers (electrospinning) from both natural and synthetic materials. The fibers produced through electrospinning present larger surface area than the ones obtained by traditional spinning methods as they have a thin diameter, ranging from a few nanometers to many micrometers (27,28). The coaxial electrospinning technique is a new, innovative process for encapsulation in micro- and nanoscale. With this technique, bioactive ingredients can be encapsulated in selected polymeric matrixes to develop micro- and nanofibers with specific morphology (fiber thickness and diameter, concentration of compounds, etc.) to achieve the desirable properties (e.g., controlled release, solubility, etc.) (29). To commercialize development, the cosmetic industry must ensure that products maintain some basic quality characteristics. Thus, several tests regarding their stability, safety, sensory characteristics, and reported actions have to be performed (14). One major concern when a new cosmetic product is introduced to the market is the alterations that may occur after its manufacturing (e.g., physicochemical, chemical, physical, and microbiological) that determine its expiration date. Microbial contamination and growth may cause infection on the skin. In addition, these alterations may also destroy the nature of cosmetics due to changes in viscosity and characteristics such as color, odor, and emulsion stability (7,30). Cosmetic formulations are mainly contaminated from Staphylococcus aureus, Salmonella species (spp.), Pseudomonas aeruginosa, and Escherichia coli (31). Cosmetic creams exhibit a slightly acidic pH near normal skin surface pH, ranging between 4 and 6. It has been reported that facial skin product pH has a big impact on its efficacy. The formulation of more neutral pH products present decreased efficacy but can be useful when formulating for sensitive skin types. There are limitations regarding the range of acceptable pH value, since it is a crucial parameter that affects the incorporated ingredients. In a cosmetic system, the incorporated ingredients may be incompatible when mixed together and may be easily affected by changes in pH (e.g., polymeric thickeners, dyes, and certain preservatives). Thus, pH is an important parameter that should be stable during the whole life of the product (32). Α cosmetic product should remain stable thought its life time, meaning the expected period of usage by the consumer as well as to fulfill the user’s requirements. To ensure that a product fulfills the required criteria of safety, quality, and effectiveness, cosmetics should be tested at various recommended temperatures and time periods to observe and measure property changes over time (33). This study examined the incorporation of encapsulated TTO in a cosmetic facial cream (incorporation in basic formulation) and the quality characterization and shelf-life evaluation of the formulated product to ensure the newly proposed formulation would not affect the cream in a negative way. The encapsulation of antiseptic TTO extract in β-cyclodextrin’s polymeric matrix nanofibers was performed through the electrohydrodynamic process. Cosmetic facial creams without (control samples) TTO and with TTO nanofibers were stored at three different temperatures. Subsequently, microbiological analysis, color and pH measurements were measured for the control cream and the cream containing TTO nanofibers.
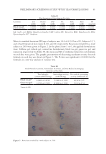
73 ENCAPSULATED TTO IN FACIAL CREAMS MATERIALS AND METHODS MATERIALS Aqueous 60% β-cyclodextrin solution (hydropropyl-beta-cyclodextrin, HPbCD, I.R.A. Istituto Ricerche Applicate, Italy) and TTO (Main Camp Natural Extracts Pty Ltd, Australia), commercially labeled as “PHYTODERMINA LIFTING” and “Tea Tree Oil (M alternifolia)” respectively, were supplied by Cellco Chemicals SA. ELECTROHYDRODYNAMIC PROCESS (ELECTROSPINNING) The electrohydrodynamic process was performed in the electrospinning apparatus FluidNatek®, equipped with a variable high-voltage 0–30 kV power supply (Bioinicia S.L., Valencia, Spain). Cyclodextrin solution (matrix) and TTO (encapsulated material) were electrospun in a horizontal coaxial electrospinning setup. Cyclodextrin and TTO were placed in a different plastic syringe (10 mL, 14.22 mm diameter) each, with horizontal orientation on a pump that digitally control them, with their needles vertically directed toward the collector. The electrode emitting positive polarity of the high-voltage power supply were connected to the needles and an aluminum foil sheet was attached to a copper grid, placed 15 cm from the capillary tip, used to collect the electrospun fibers. The electrospinning experiments were carried out at room temperature in air and the flow rate of cyclodextrin solution was set to 0.5 mL/h and of TTO to 0.4 mL/h, while the voltage was set to 26.1 kV. In optimum conditions the encapsulation efficiency was 75% and the amount of TTO was extracted from weighted nanofibers (collected after 2 hours) using ethanol as solvent. The encapsulation efficiency was then determined using a UV– spectrophotometer at 225 nm. SCANNING ELECTRON MISCROSOCPY Morphology of TTO encapsulated microfibers was evaluated using scanning electron microscopy (SEM) (Quanta 200, FEI Oregon, USA, voltage 12.5 kV, LFD detector, Spot size 4.5, magnification ×12000). Prior to SEM, a 13.23 nm coat of gold layering was applied to make the surface reflect the electron beam (SC7620 Mini Sputter Coater, Quorum Technologies, West Sussex, UK /105 s, 18 mA, 1KV). PREPARATION OF FACIAL CREAM SAMPLES The facial cream was based on a natural olive-oil–derived emulsifier and a natural wax containing 12% natural and synthetic oils stabilized by natural polymers (carrageenan, cellulose gum, xanthan gum) (sample C). Sample C did not contain TTO. For the formulation of the samples containing encapsulated TTO (sample T) the required quantity of powdered encapsulated fibers were added in the basic cream to achieve 0.5% TTO quantity in the final formulation. The percentage of TTO in the final encapsulated fibers was approximately 37%.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)