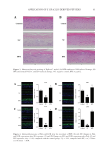
74 JOURNAL OF COSMETIC SCIENCE STORAGE CONDITIONS Immediately after their formulation (time zero, control samples), facial creams were sampled in the laboratory and stored in glass containers in temperature-controlled cabinets at three selected storage temperatures: 5°C, 25°C, and 45°C and analyzed to verify their stability over storage time. A storage temperature of 45°C was selected to facilitate an accelerated shelf-life testing protocol and all samples were stored for 50 d in total. For all tests cream sampling was performed after their formulation (time zero, control samples) and after 30 and 50 d of storage in all the different temperatures. MICROBIOLOGICAL ANALYSIS For microbiological enumeration, a representative sample of 1 g of each sample was aseptically taken from each product and placed in 9 mL sterilized Ringer solution (Ringer tablet, Merck code 1.15525, Darmstadt, Germany), thus giving a 10:1 dilution. Samples were homogenized using with a vortex at room temperature. The outside surfaces of all containers were swabbed with ethanol (70% v/v) before opening. Sampling of the creams was carried after 30 and 50 d of storage. The microbiological stability of all products was examined. Total count and yeasts/molds (YM) were determined, as well as the pathogens, S aureus, P aeruginosa, E coli, and Salmonella spp. Compact dry plates, a ready-to-use test method, was selected for the microbiological analysis. For all plates, typical colony types and morphology characteristics associated with each growth medium were examined. The number of viable germs in a culture can be ascertained by determining the number of colonies forming units (cfu/g), with the colony counting technique. Plate reading and colony count were reported in cfu/g (34). TOTAL BACTERIAL COUNT Compact Dry TC is a medium contains nutrient standard agar, suitable for total viable bacterial count. Aerobic bacterial colony counts were made by spreading 1 mL of each 10:1 diluted sample on Compact Dry TC medium for total count (Compact Dry TC, Hyserve GmbH &Co. KG, Uffing, Germany) and incubated at 37°C for 48–72 hours. Each assay was performed in duplicate. After incubation, the number of colonies was recorded for each plate. In samples with low counts, the count was recorded as 100 cfu/g of product. YEASTS/MOLDS YM are both fungal spp. and many, as with other microorganisms, can cause serious infections of the immunocompromised. YM can be differentiated by color development. A ready-to-use plate labeled Compact Dry YM contains a medium with a chromogenic enzyme substrate (XPhos) that changes to blue when many of the yeasts are detected. During the 3-dimension growth of yeast and mold, fluffy colonies with characteristic colors are formed while antibiotics inhibit the growth of bacteria. For the enumeration of YM 1 mL of each 10:1 diluted sample was spread on Compact Dry YM ready-to-use plates for yeast and mold (Compact Dry YM, Hyserve GmbH &Co. KG, Uffing, Germany) after
75 ENCAPSULATED TTO IN FACIAL CREAMS incubation at 30°C for 3–5 d in the dark. Samples with low counts were recorded as 10 cfu/g of product. Media and enumeration of pathogenic microorganisms. Pseudomonas spp. are very widespread in nature particularly in water. For the enumeration of P aeruginosa pathogens, which are generally infecting immunocompromised patients, 1 mL of each 10:1 diluted sample was spread on Compact Dry Pseudomonas (PA) ready-to-use plates for Pseudomonas (Compact Dry PA, Hyserve GmbH &Co. KG, Uffing, Germany). Compact Dry SA is a medium to determine S aureus by means of selective growth of Staphylococcus and differentiation by egg-yolk reaction. Compact Dry SA plate is based on improved mannitol salt agar. Staphylococcus aureus generates yellow pigments that result in light-yellow colonies. The lipid–protein complex (lecithin) in the egg-yolk reaction is split by lipase which changes the peripheral medium around the colonies to turbid-white. For the isolation and enumeration of S aureus in samples, 1 mL of each 10:1 diluted sample was spread on Compact Dry SA ready-to-use plates for S aureus (Compact Dry SA, Hyserve GmbH &Co. KG, Uffing, Germany). Plates were incubated at 37°C for 48 h for the enumeration of the spp. Escherichia coli spp. belong to the coliform group of bacteria. This bacterium can be distinguished from other coliforms by its growth and color reaction on certain types of culture media. Escherichia coli is a pathogen microorganism of great concern because it can be found in the water. Water is basic compound used in various industries and products, including pharmaceuticals, dietary supplement, nutraceuticals, cosmetics, and toiletry industries. Microbiological requirements ensure the absence of coliforms. To determine the presence of E coli, 1 mL of each 10:1 diluted sample was spread on Compact Dry E coliready- to-use plates for E coli and coliforms (Compact Dry EC, Hyserve GmbH &Co. KG, Uffing, Germany). The plates were incubated at 37°C for 24 h for the enumeration of the spp. Compact Dry SL plates contain a selective growth medium used in the isolation of Salmonella spp. from clinical samples and food. The plates are based on the combination of three different test principles: (1) alkalization of the medium by Salmonella’s lysine decarboxylase ability (medium color will change blue–purple to yellow) (2) greening colony caused by decomposition of chromogenic substrate with specific enzyme of Salmonella (black colonies are generated by hydrogen sulfide producing Salmonella) (3) motility of Salmonella. Compact Dry SL allows a much more rapid screening for Salmonella therefore, for the enumeration of Salmonella spp., 1 mL of each 10:1 diluted sample was spread on Compact Dry SL ready-to- use plates for Salmonella (Compact Dry SL, Hyserve GmbH &Co. KG, Uffing, Germany). The plates were incubated at 37°C for 24 h for the enumeration of the spp. pH MEASUREMENTS The pH values of all creams were measured using a pH meter (Multiparameter Bench Meter, Mi 180, MARTINI instruments). The pH meter was calibrated using standard buffer solutions, 1 g of each sample was diluted in 9 mL Ringer’s solution (1:10 dilution), and its pH was recorded. COLOR MEASUREMENTS A specific amount of each sample was poured into the measurement cell and analyzed. Color of all samples was measured with the color meter MiniScan XE (Hunter Associates
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)