86 JOURNAL OF COSMETIC SCIENCE MATERIALS AND METHODS PREPARATION OF BIOACTIVE PEPTIDES Euglena gracilis (FACHB-848) was purchased from the Freshwater Algae Culture Collection at the Institute of Hydrobiology (Wuhan, China) and then cultured in an autotrophic medium (AF-6) with a controlled temperature and light exposure (25°C, light/dark =12/12, 30 μm photons m−2 s−1) (15). In the exponential phase, E gracilis cells were collected and washed with distilled water. Cells were then broken by an ultrasonic method, and its pH was adjusted to approximately 7.0–8.0. The crude extract was centrifuged at 4°C with 10,000 g for 30 min. The protein was precipitated at pI. The pH of the supernatant was then briefly decreased to 4.0 progressively using hydrochloric acid (16). The protein precipitation was collected by centrifugation and redissolved in double distilled water at pH of 7.0. After determining the protein concentration using the Coomassie brilliant blue method (0.54 mg/mL), the protein solution was hydrolyzed by a commercial neutral protease (Beijing Solarbio Science &Technology Co., Ltd., Beijing, China). The hydrolysis process was conducted with a 0.1% neutral protease under 50°C for 4 h. The enzymatic peptides were then ultrafiltered with a 3 kD ultrafiltration membrane and stored at −80°C for the next experiments. PREPARATION OF CELLS AND 3D EPIDERMAL SKIN MODEL HaCaT and RAW 264.7 cells, purchased from Fenghui Biology (Hunan, China), were maintained in Dulbecco’s modified Eagle medium (DMEM) solutions (Wisent, China), which were supplemented with 10% (vol/vol) fetal bovine serum (Invitrogen, USA) and 1% penicillin/streptomycin (Lanke Biology, China, 100 IU/mL). Primary culture cells (HDF), purchased from Beina Biology in China, was also cultured in low-glucose DMEM solutions (Wisent, China), which were also supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY) and 1% penicillin/streptomycin (Lanke Biology, Hangzhou, China, 100 IU/mL). 3D epidermal skin model Epikutis® (Zhengzhou Biocell Biotechnology Co., Ltd. Henan, China) was maintained in EpiGrowth solutions. Cell and Epikutis® were cultured at 37°C in a 5% CO 2 -containing humidified atmosphere. ASSAY OF CELL VIABILITY Immortalized keratinocytes (HaCaT cells) were cultured in 96-well plates for 24 h at 4 × 104 cells/mL (100 μL/well). Then, HaCaT cells were treated with 100 mg/L of sodium lauryl sulfate (SLS, Sigma, USA) and supplemented with 1% BPE for 24 h. The positive controls were treated with 0.1% of Comthing® SGS (ComThings SAS, Immeuble Premium, Nice, France), a commercial product (http://greaf-cc.ecer.com/sale-9601118-comthing-sgs.html). After incubation, HaCaT cells were washed by PBS three times, and cell viability was determined using the Cell Counting Kit-8 Assay reagent (Dojindo, Japan). ASSAY OF REACTIVE OXYGEN SPECIES (ROS) Human dermal fibroblasts (HDF cells) were cultured in 96-well plates at 1 × 104 cells/mL (100 μL/well) for 48 h. The cells were then treated with 10 μm of hydrogen peroxide (H 2 O 2 ,
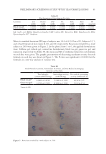
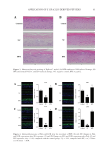
87 APPLICATION OF E GRACILIS-DERIVED PEPTIDES Sigma, USA) for 2 h. H 2 O 2 -treated cells were washed twice with PBS and incubated in DMEM solutions for 24 h. The positive controls were treated with 100 μg/mL of Vitamin E and experimental groups were treated with 5 μg/mL of BPE. For ROS analysis (17), 10 μm of 2’,7’-dichlorofluorescein diacetate (DCFH-DA, Beyotime, China) was added in the cell suspension at 37°C for 30 min in darkness. The intensity of fluorescence was measured with a fluorescent microplate reader (Molecular Devices, USA). ASSAY OF INFLAMMATORY RESPONSE The Mouse tumor necrosis factor α (TNF-α) enzyme linked immunosorbent assay (ELISA) Kit (Multi, China) and Human thymic stromal lymphopoietin (TSLP) ELISA Kit (Boster, China) were used to measure the content of extracellular TNF-α and TSLP (18,19). In brief, RAW 264.7 cells were cultured in 96-well plates at a density of 2 × 104 cells per well. After 24 h of cultivation, 1 mg/L of lipopolysaccharides (LPS) (Sigma, USA) was used to stimulate inflammatory response of cells. Meanwhile, the experimental groups were supplemented with 1% BPE for 24 h, and the positive controls were treated with 100 μg/mL of dexamethasone (DXMS). The extracellular medium was then collected to determine TNF-α. The absorbance value was detected at 450 nm, and the enzyme activity of TNF-α in the sample was calculated according to a standard curve. The culture medium of Epikutis® was also collected to analyze the inflammatory response. TSLP was measured using Human TSLP ELISA Kit at 450 nm with a microplate reader (BioTek, USA). To explore the changes in gene expression, transcriptome analysis was conducted. LPS- induced RAW 264.7 was briefly incubated with BPE in 6-well plates for 24 h. After being washed by 1× phosphate-buffered saline, RNA was extracted with a TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. cDNA was then synthesized and amplified to construct sequencing libraries using LianChuan Biology (Hangzhou, China). Graphene oxide enrichment analysis referred to the website David (https://david. ncifcrf.gov/summary.jsp), and the heatmap was analyzed via the website Morpheus (https:// software.broadinstitute.org/morpheus/). 3D EPIDERMAL SKIN MODEL The 3D epidermal skin model (Epikutis®) was cultured in 6-well plates with EpiGrowth solutions. After treatments with Poly I:C (Sigma, USA), LPS (Sigma, USA), and SLS (Sigma, USA), Epikutis® was fixed with 4% polyformaldehyde for 24 h. Afterward, the samples were dehydrated, embedded in paraffin, and cut into 5 μm sections. After hematoxylin and eosin staining, the sections were dried at room temperature and then mounted with neutral gum. Finally, the morphology of Epikutis® was examined under a microscope (Leica, German) (19). Immunofluorescence analysis was performed as follows (20): the paraffin sections were put into xylene for 10 min and hydrated with anhydrous and 95% ethanol after rinsing with distilled water, the sections were put into 0.01 M of sodium citrate with high pressure and supplemented with one drop of 3% H 2 O 2 for 30 min, which could block the activity of endogenous peroxidase the sections were then incubated with anti- filaggrin (FLG) or anti-loricrin (LOR) (Abcam, USA) at 4°C overnight anti–Mouse-488 (Abcam, USA) was
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)