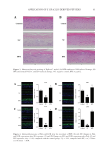
88 JOURNAL OF COSMETIC SCIENCE used as a secondary antibody for 1 h of incubation and the sections were finally observed by fluorescence microscopy (Leica, German). Analysis of immunofluorescence intensity was performed in a blinded manner. CLINICAL TRIALS A double-blind clinical trial was conducted to evaluate the changes in allergic inflammation. The clinical trial consisted of 16 volunteers aged 20–58 years old (4 males and 12 females). In brief, 4 cm2 areas on the surface of one forearm was provoked with 10 mg/mL of histamine dihydrochloride. After provocation, double distilled water and a 5% BPE solution were gently rubbed on the targeted area. Erythema, which reflects the degree of inflammation, was evaluated by a Cutometer Dual MPA 580 with a probe Colorimeter CL 400 (Courage and Khazaka Electronics, Cologne, Germany) and expressed in the L*a*b* system. The a* value on the green–red axis was used to assess the erythema. All measurements were performed under controlled environmental conditions (room temperature of approximately 20.1–22.8°C and an average relative humidity of approximately 41.7–60.4%). All patients underwent subjective evaluation of their itch using a customer questionnaire (21). STATISTICAL ANALYSIS All data were presented as the mean of three individual experiments. Significant differences were calculated using student’s t-test via GraphPad Prism 8 software. Differences were considered statistically significant when p values 0.05. RESULTS AND DISCUSSION 2D SKIN MODELS Allergic inflammation was simulated through chemical induction in 2D skin models. The efficacy of BPE for dermatitis treatment was evaluated based on these models. HaCaT cells were chosen to examine the cell protection function of BPE (22). Figure 1A illustrated that cell viability significantly decreased after SLS exposure. The negative control reached 41%, but it was improved by the treatments with anti-inflammation pharmaceuticals and BPE, which were 62 and 55% respectively. ROS level is an important indicator during oxidation reaction (23,24). Figure 1B showed that ROS levels were 41 and 68% for the vitamin E and BPE treatments. The antioxidant activity of BPE is similar to other algal peptides (25). It implied that BPE performed a potential function in alleviating oxidative damages. Because of this, the results indicated that BPE played an effective role in the process of cell protection. Expression of TNF-α is sensitive to inflammatory response (26,27). Changes in TNF-α contents were analyzed to evaluate inflammatory response. As shown in Figure 1C, LPS exposure significantly increased the TNF-α content of RAW 264.7 cells, which indicated a remarkable inflammatory response. After DXMS and BPE treatments, TNF-α contents significantly decreased (Figure 1C), indicating BPE diminished inflammation. In all, 2D skin models verified that BPE exerted antioxidant and anti-inflammatory activity.
89 APPLICATION OF E GRACILIS-DERIVED PEPTIDES TRANSCRIPTOME ANALYSIS Transcriptome analysis was performed to reveal the changes in gene expression of RAW 264.7 cells under the function of BPE. Herein, LPS was used to induce the inflammatory response of cells, and dexamethasone treatment was applied as the positive control. As shown in Figure 2, LPS simulation induced specific changes in gene expression, characterized with 1,215 upregulated genes and 809 downregulated genes in the negative groups. Among these genes, inflammation- and immune response-related genes such as tumor necrosis factor superfamily member 10 (TNFSF10), interferon-induced protein 44-like gene (IFI44L), discs large homolog 5 (DLG5), and calcium-binding mitochondrial carrier protein gene (SLC25A24) were significantly regulated, indicating the inflammatory and immune responses simulated by LPS. TNFSF10 is known as a TNF-related apoptosis-inducing ligand (TRAIL). TRAIL’s downregulation in all LPS- simulated groups suggested the original cell-preventive effect against cell death (28). IFI44L negatively modulates inflammation responses (29), and its downregulation indicated an anti-inflammatory process. In the positive control and BPE treatment, DLG5, and SLC25A24 were significantly upregulated compared to the negative group. DLG5 encodes a scaffolding protein that is involved in the maintenance of epithelial integrity (30). It was observed to be a susceptibility gene for inflammatory bowel disease, which is an archetypal inflammatory barrier disease (31). SLC25A24 is a kind of antiapoptotic protein gene, which is important in the response to oxidative stress (32,33). Therefore, BPE enhanced the anti-inflammatory ability of the cells. 3D SKIN MODELS A 3D skin model, Epikutis®, was chosen to evaluate the relief of allergic inflammation via BPE treatments. Multiple techniques (e.g., hematoxylin-eosin staining, immunofluorescence, and ELISA) were applied in the simulation experiments. As shown in Figure 3A, the damage induced by Poly I:C and LPS was significantly improved when Epikutis® was treated with BPE for 24 h. Meanwhile, BPE also alleviated the damage induced by SLS (Figure 3B). Figure 1. Effects of BPE on 2D cell models. (A) Assay of cell viability by HaCaT cells. (B) Assay of ROS level. (C) Assay of TNF-α content by RAW 264.7 cells. NC: negative control SGS: positive control, 0.1%, a commercial product (http://greaf-cc.ecer.com/sale-9601118-comthing-sgs.html) Vitamin E: positive control, 100 μg/mL DXMS: positive control, dexamethasone, 100 μg/mL BPE: 5 μg/mL #p 0.05, ##p 0.01, ###p 0.001, compared with the control group **p 0.01, ***p 0.001, compared with NC, n =6. Values are the mean ± SEM.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)