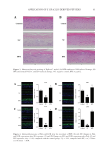
80 JOURNAL OF COSMETIC SCIENCE Table IV Color Parameters ∆E, L*, a*, and b* of C and T Cream Samples Stored at 5°C Samples Storage time (d) ∆E L* a* b* C 0 0.00 66.56 –1.41 5.23 30 0.60 65.96 –1.35 5.21 50 0.44 66.83 –1.55 4.91 T 0 0.00 66.27 –1.40 5.24 30 1.42 65.00 –1.26 4.63 50 0.54 66.73 –1.54 4.99 Table V Color Parameters ∆E, L*, a*, and b* of C and T Cream Samples Stored at 25°C Samples Storage time (d) ∆E L* a* b* C 0 0 66.56 –1.41 5.23 30 0.87 67.42 –1.5 5.13 50 0.51 66.83 –1.55 4.82 T 0 0 66.27 –1.4 5.24 30 1.78 64.63 –1.47 4.56 50 1.03 67.16 –1.66 4.80 Table VI Color Parameters ∆E, L*, a*, and b* of C and T Cream Samples Stored at 45°C Samples Storage time (d) ∆E L* a* b* C 0 0.00 66.56 –1.41 5.23 30 0.81 66.99 –1.58 4.56 50 0.13 66.67 –1.44 5.29 T 0 0.00 66.27 –1.40 5.24 30 0.91 65.39 –1.63 5.25 50 0.92 67.14 –1.67 5.39 Figure 3. ΔE values of a C and T samples stored at 5°C, 25°C, and 45°C.
81 ENCAPSULATED TTO IN FACIAL CREAMS CONCLUSION This study has raised awareness of important aspects of the incorporation of encapsulated TTO in facial cosmetic creams. The selected conditions of encapsulation through the electrohydrodynamic process produce homogeneous fibers. Since no microbial growth occurred, all the formulated creams analyzed were microbiologically stable and free of both spoilage and pathogenic bacteria, were recorded. In addition, color and pH measurements indicated that the formulations present similar quality characteristics that are not affected by time storage. Within the limits of this study, it can be concluded that incorporation of TTO in facial cosmetic creams leads to safe products in terms of microbial growth and acceptable characteristics because the measured properties remained stable. These conclusions are primarily attributed to the suitable encapsulation technique followed to properly produce TTO structures. In fact, the final cream formulations with encapsulated TTO do not present undesirable changes in color, odor, or emulsion stability they are microbiologically stable and present similar properties with the creams without TTO during storage. In terms of their microbiological aspects and quality characteristics, the suggested formulations are suitable for use as cosmetic products. The encapsulation method used in this study, the electrohydrodynamic process, could be applied to encapsulate several plant extracts to overcome the numerous difficulties presented during their incorporation in cosmetic products. Usually, these problems refer to either undesirable color or odor of the plant extracts or in formulation issues due to their unwanted lipophilic or lyophobic character. The electrohydrodynamic process could be the key for encapsulated formulations with desired structure and properties without damaging the valuable thermosensitive compounds usually selected as additives in cosmetic products. REFERENCES (1) W. A. Żukiewicz-Sobczak, P. Adamczuk, P. Wróblewska, J. Zwoliński, J. Chmielewska-Badora, E. Krasowska, E. M. Galińska, G. Cholewa, J. Piątek, and J. Koźlik, Allergy to selected cosmetic ingredients, Postepy Dermatologii i Alergologii. 30(5), 307–310 (2013). (2) E. M. Warshaw, H. J. Buchholz, D. V. Belsito, H. I. Maibach, J. F. Fowler, R. L. Rietschel, K. A. Zug, C. G. Mathias, M. D. Pratt, D. Sasseville, F. J. Storrs, J. S. Taylor, V. A. Deleo, and J. G. Marks, Allergic patch test reactions associated with cosmetics: retrospective analysis of cross-sectional data from the North American Contact Dermatitis Group, 2001–2004, J. Am. Academy of Dermatol., 60(1), 23–38 (2009). (3) R. Wolf, D. Wolf, B. Tüzün, and Y. Tüzün, Contact dermatitis to cosmetics, Clinics in Dermatol., 19(4), 502–515 (2001). (4) L. Leistner, Basic aspects of food preservation by hurdle technology, Int. J.Food Microbiol., 55(1–3), 181– 186 (2000). (5) S. Papageorgiou, A. Varvaresou, E. Tsirivas, and C. Demetzos, New alternatives to cosmetics preservation, J. Cosmet. Sci., 61(2), 107–123 (2010). (6) A. Varvaresou, S. Papageorgiou, E. Tsirivas, E. Protopapa, H. Kintziou, V. Kefala, and C. Demetzos, Self-preserving cosmetics, Int. J. Cosmet. Sci., 31(3), 163–175 (2009). (7) S. Chouhan, K. Sharma, and S. Guleria, Antimicrobial activity of some essential oils-present status and future perspectives, Medicines. 4(3), 58 (2017). (8) P. S. X. Yap, B. C. Yiap, H. C. Ping, and S. H. E. Lim, Essential oils, a new horizon in combating bacterial antibiotic resistance, Open Microbiol. J., 8, 6–14 (2014).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)