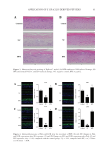
76 JOURNAL OF COSMETIC SCIENCE Laboratory Inc, Reston, Virginia) using the Commission International de l’ Eclairage color system (L*, a*, b*) (CIE 041-1978, Light as a true visual quantity: Principles of measurement, STANDARD by Commission Internationale de L’Eclairage, 01/01/1978). Lightness (L*), with values from 0 (black) to 100 (white), represent the central vertical axis. On the a–a’ axis, positive values indicate amounts of red, while negative values indicate amounts of green. On the b–b’ axis, yellow is positive, and blue is negative. The color axes is used because a color cannot be both red and green, or both blue and yellow, as these colors oppose each other. Values range from positive to negative on each axis. Before measurements, the color meter is calibrated with a white and black calibration plate. The measured color changes are expressed in total as ΔE. The color of a cream was measured on the first day of sampling as a reference. ΔE is the total color change calculated from the following equation: ∆ ∆L ∆ ∆b*)2] E (a* )(=++[*)2 (2 1 2 ∆E =total color change RESULTS &DISCUSSIONS The results obtained from the microbiological analysis of all samples for 50 d storage appear in the Tables I–III. Results revealed that the formulated creams analyzed were microbiologically stable, since no microbial growth (both spoilage and pathogenic bacteria) were recorded during the 50 d storage of the samples. Moreover, results showed that the storage temperature of the samples (5°C, 25°C, and 45°C) and the addition of the encapsulated TTO did not affect the microbial load of cosmetic creams. The results from the microbial analysis were negative, confirming that all the examined samples met the requirements specified for human usage (35,36). Yeast and molds have often been examined in cosmetic products to assess sanitation conditions, microbiological safety, and product quality during processing and storage (37). Cosmetic formulations are good mediums for microbial growth due to components like water, various minerals and vitamins, and also provide oxygen, favorable pH, and temperature (31). In addition, cosmetic creams being potentially exposed to contaminants during manufacture and usage (incorrect use, hygiene of the hands, etc.) is quite common. Thus, a major concern for the cosmetics industry is the development of formulations to prevent microbial growth (38,39). Table I Counts of Bacteria Associated With Cosmetic Creams Stored at 5°C for 50 d Sample Total bacterial count YM P aeruginosa S aureus E coli Salmonella C 2 log cfu/g 1 log cfu/g absence/g absence/g absence/g absence/g T 2 log cfu/g 1 log cfu/g absence/g absence/g absence/g absence/g Table II Counts of Bacteria Associated With Cosmetic Creams Stored at 25°C for 50 d Sample Total bacterial count YM P aeruginosa S aureus E coli Salmonella C 2 log cfu/g 1 log cfu/g absence/g absence/g absence/g absence/g T 2 log cfu/g 1 log cfu/g absence/g absence/g absence/g absence/g
77 ENCAPSULATED TTO IN FACIAL CREAMS An important contamination of the cosmetics during usage refers to Staphylococcus spp. mainly due to the contact with users’ hands as presented by Campana et al. (34). The recovery of these potential pathogenic spp. suggested that the preservative system was not effective, in contrast to the samples of the present study, where it is apparent that they were not contaminated during storage, due to proper handling and storage conditions. Behravan et al. reported the need for a system to prevent microbial growth (38). They investigated the presence of microorganisms in a variety of sealed and used cosmetic creams and found that the contamination with Gram-positive bacilli, S aureus, was much greater in all samples, while some Gram-negative E coli were also isolated from both the used and unused creams. They were found to be higher for used cosmetic creams (45%, 38%, and 8%, respectively) than for unused creams (38%, 25%, and 0%, respectively). In addition, 17% of the unused creams were found to not be viable microorganisms, while only 10% of the creams used did not contain viable microorganisms. Most of the studies did not report on Salmonella spp. infection due to use of contaminated cosmetic preparations, which was the case in our study. Specifically, this could be attributed to several compounds (especially plant origin) that are commonly used in preparation of cosmetics and prevent Salmonella spp. growth (40). Table III Counts of Bacteria Associated With Cosmetic Creams Stored at 45°C for 50 d Sample Total bacterial count YM P aeruginosa S aureus E coli Salmonella C 2 log cfu/g 1 log cfu/g absence/g absence/g absence/g absence/g T 2 log cfu/g 1 log cfu/g absence/g absence/g absence/g absence/g Figure 1. SEM of encapsulated TTO microfibers.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)