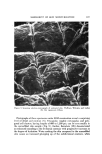
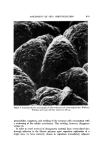
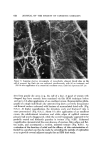
592 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 6.0 per cent thioglycolic acid adjusted to pH 9.2 with ammonia. In a separate set of experiments fibers were also treated with either 0.5 per cent or 6.0 per cent thioglycolic acid at pH 11.3 (with sodium hydroxide) for 9. or 5 min. Only exposure to 6 per cent thioglycolic acid at pH 11.3 for 5 min effected any lowering of the cutting force over that of water. The majority of the fibers with this treatment disintegrated before they could be cut, and the two fibers which could be cut bent during cutting. The conclusions, •vhich must be drawn from these studies, are that even the most severe chemical (covalent bond) damage, which is knoven to lower the tensile modulus drastically, has almost no effect on the force required to cut beard hair. In addition, rupture of hydrophobic bonds by 1-propanol/ water also appears to have almost no effect on the f-t-c. CONCLUSION A device is described which permits measurement of the force required to cut a beard hair fiber under a variety of conditions. Studies with this device show that the force required to cut wet beard fibers with commercial razor blades is about 65 per cent less than that of dry fibers. Beard hair is almost completely hydrated by exposure to water for about 2 min at room tempera- ture, and this hydration is accelerated by an in,crease in temperature. The force required to cut beard hair is not lowered below that of water by the presence of a wetting agent, shaving cream, or soap solution. The force required to cut wet beard hair with a razor blade is lowered significantly by verv severe chemical attack on the fiber. On the other hand, the force re- quired to cut beard hair increases as the rate of blade travel increases. ( Received December 3, 1975 ) I•EFERENCES (1) L. Hollander and E. 1. Casselman, Factors involved in satisfactory shaving, ]. Amer. Med. Assoc., 109, 95-101 (1937). (2) A. Dupr6, Cinqui6me M6moire sur la Th6orie M•canique de la Chaleur, Travail et Force Moleculaires II, Ann. Chim. Phys., 7, 236-82 (1866). (3) N. I. Muskhelishoili, Some Basic Problems of Mathematical Theory of Elasticity (trans- lated from the Russian by J. R. M. Radok), P. Noordoff Ltd., Gronigen, Holland, 1953. (4) Mary G. Natrella, Experimental Statistics, U.S. Govt. Printing Office, N.B.S. Hand- book 91, 1963, P. 5-24. (5) T. W. Mitchell and M. Feu•helman, The Torsional Properties of Single Wool Fibers, Part I, Text. Res. J., 3,0, 662-7 (1960). (6) J. C. Atkinson et al., Action of Mixed Solvents on Wool, Nature, 184, 444 (1959). (7) J. A. MacLaren, The extent of reduction of wool protein by thiols, Aust. J. Chem., 15, 824-31 (1962).
J. Soc. Cosmet. Chem., 27, 593-605 (December 1976) Methoden zur Bestimmung von Fluoridionen in Zahnpasten II. Studie zur Trennung der zu bestimmenden Ionen durch Destillation mit tiberhitztem Wasserdampf M. O. SCHMITZ-MASSE**, M. HANOCQ* und M. HERPOL-BORREMANS** Synopsis -- Methods for the Determination of Fluoride Ions in Toothpastes. II: Study of the Separation of the Ionic Species of Interest with the Aid of St, per-heated Systems. -- In the first part of this study the conditions for the separation of fluoride ion in toothpastes are examined. The method which depends on the codistillation of hexafluorosilicic acid with super-heated steam, vehich is generated in a suitable apparatus, has proved itself useful. The procedure is independent of the method of preparation of the toothpaste and of the type of fluoride derivative present in the product. The second part is a comparison of two assay procedures. The first one -- a spectrophotometric procedure -- utilizes formation of a complex between cerium***, alizarin, Complexon, and fluoride. The other -- a potentio- metric assay -- depends on the use of a lanthanum fluoride membrane electrode. From the point of view of sensitivity, reproducibility, and precision the two methods are comparable. Einleitung Im Verlauoe des ersten Tells der vergleichenden Arbeit (1) haben wir gezeigt, daf• die Bestimmung des Aluminiumfiuorids nach seiner Abtrennung yore Anion dutch Mikrodiffusion nicht quantitativ war. Unser Ziel bei der Inangriffnahme dieset neuen Arbeit ist es, eine brauchbare Bestimmungsmethode yon Fluoridionen in Zahnpasten vorzuschlagen, und * Laboratoire de Chimie Analytique, Chimie Pharmaceutique inorganique et de Toxicologie (Professeur L. Molle). Institut de Pharmacie, Universit• Libre de Bruxelles, Campus de la Plaine, 1050 Bruxelles. ** Minist•re de la Sant• Publique. Institut d'Hygi•ne et d'Epid•miologie (Professcur Dr. Lafontaine). D•partement Pharmaco-Toxicologie. 14, rue J. Wytsman, 1050 Bruxelles. 593
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)