j. Soc. Cosmet. Chem., 31,273-278 (September/October 1980) A replacement for Rubine dye for detecting cationics on keratin RICHARD J. CRAWFORD and CLARENCE R. ROBBINS, Colgate Palmolive Research Center, Piscataway, NJ 08854. Received February 12, 1980. Synopsis Four dyes have been examined as possible substitutes for RUBINE DYE to test for the presence of CATIONIC SURFACTANTS and POLYMERS on hair and wool. The four dyes tested were Red 80 (Sirius Red), Red 84 (Pyrazol Fast Red), Orange II and Orange G. Red 80 proved to be an excellent substitute for Rubine. INTRODUCTION Rubine dye (1) (Structure I) has been used as a stain for detecting cationic surfactants and polymers on keratin substrates (2), however it is no longer being manufactured. Therefore, we elected to search for a suitable stain to replace it. A few dyes similar in structure to Rubine were located in the Color Index (3), from which we selected Red 80 (4) (Structure, IX) and Red 84 (5) (Structure Ill) to examine experimentally. These two dyes are related structurally to Rubine, all three being high molecular weight, polyaromatic, symmetrically disubstituted ureas, containing four to six sulfonate groups and four azo linkages. -- -- 803Na • OH NaOaS N - I H 2 Rubine Dye (Mol. Wt. 1472) SOaNa -o- -c5- NaOaS N•N N=N NaOaS.-'-'-•'•. N / I H -- Red 80 (Mol. Wt. 1372) /c=o 273
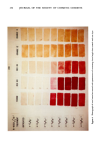
274 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Na03S•• -N:N N:N--•i-- Na03S Red 84 (Mol. Wt. 1136) --C-----O EXPERIMENTAL Quaternary ammonium compounds were synthesized with the exception of dodecyl (6) and hexadecyl (6) trimethyl ammonium bromides. All these structures were confirmed by NMR. Wool (7) and hair (8) swatches were treated with these quaternary ammonium compounds using 10 millimolar solutions and a 20:1 solution-to-swatch ratio for 2 minutes. Treatment with commercial products was with a 1:1 product- to-swatch ratio. All swatches were rinsed 5 times using a 50:1 solution-to-swatch ratio. Stock solutions of the dyes were made using 3.4 millimoles of dye (assuming 100% purity) and 1.25 ml of glacial acetic acid per liter of water, and diluted 5:1 with water for treatment of the quaternary treated swatches. A 1-minute treatment time and a 20:1 solution to swatch ratio was used. Dye treated swatches were rinsed 5 times using a 50:1 solution-to-swatch ratio. Stain intensity readings were taken using a Gardner Reflectometer. DISCUSSION These dyes (II and III) were evaluated with Rubine by comparing their ability to stain wool swatches treated with a complete even carbon series of n-alkyl trimethyl ammonium bromides from butyl to octadecyl. We also decided to include two smaller, less structurally complex anionic dyes in this study, Orange II (9) (Structure IV) and Orange G (10) (Structure V), to determine if they might function to detect cationics on keratin. The observations and conclusions were similar for albino hair and N=N , S03Na Orange II (Mol. Wt. 350) (iv) HO N:N Na03S S03Na Orange G (Mol. Wt. 452) (v)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)