DETECTION OF CATIONICS ON KERATIN 277 z w o o z w ...d o o o o o =•' =•' o =•' =•' o o o
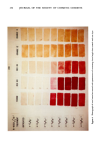
278 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS n-octyl treated swatches suggests that less cationic is on the wool, to react with dye. This is consistent with sorption studies (11), which show that short chain cationics have a lower affinity for keratin than larger cationics. This experiment suggests that Red 80 is a better replacement for Rubine, and in fact may even be superior because of its low staining on control swatches. Our final test consisted of comparing Red 80 to Rubine on wool swatches treated with several current hair products, most containing cationic conditioning agents. Figure 3 depicts the results of this experiment. Cleaning shampoos I and II are based on TEA-lauryl sulfate and ammonium lauryl sulfate respectively and neither product contains a cationic surfactant or polymer. As expected, neither of these products shows significant staining with either Red 80 or Rubine dye. On the other hand, creme rinses I and II are both based on stearalkonium chloride, and both of these products produce the expected staining reaction. Similarly, clear conditioning shampoo II contains an amine oxide and opaque conditioning shampoo contains quaternium-19, and both produce staining with either Red 80 or with Rubine. The apparent anomaly is clear conditioning shampoo I. This product contains both amine oxide and quater- nium-19 and does not show significant staining. This shampoo also contains ammonium lauryl sulfate as the primary surfactant, as opposed to the other two shampoos which produce swatch staining. Opaque conditioning shampoo is an amphoteric based system, and conditioning shampoo II is an amphoteric-nonionic based system. Apparently, these latter two shampoo systems permit deposition of cationic surfactant or polymer onto the keratin, as opposed to the anionic based shampoo which must inhibit deposition of cationic as evidenced by the lack of staining. Nevertheless, the same conclusions are permitted by use of either Red 80 or Rubine dye. CONCLUSION Since Red 80 has similar staining characteristics to Rubine with fully formulated products or with individual cationic components, we recommend it as a replacement for Rubine dye for detecting cationic conditioning agents on keratin substrates. REFERENCES (1) Direct Fast Rubine WS, C.I. No. 35790. no longer available. (2) G. V. Scott, C. R. Robbins, and J. D. Barnhurst, Sorption of Quaternary Ammonium Surfactants by Human Hair,J. Soc. Cosmet. Chem., 20, 135-152 (1969). (3) Colour Index, 3rd Edition, Volume 4, The Society of Dyers and Colorists (1971). (4) Sirius Red F3BA New, C.I. No. 35780, Verona Dyestuffs, Union, NJ Used although equivalent products are offered by Sandoz Colors & Chemicals, E. Hanover, NJ and by American Color & Chemical Corp. Charlotte, NC. (5) Atlantic Resin Fast Brown 3RL, C.I. No. 35760. Atlantic Chemical Corp., Nutley, NJ. (6) Purchased from Eastman Organic Chemicals, Rochester, NY. (7) Purchased from Test Fabric Inc. of Middlesex, NJ. (8) Purchased from De Meo Bros., 135 Fifth Ave., New York, NY. (9) Orange II, C.I. No. 15510. Eastman Kodak Co., Rochester, NY. (10) Orange G, C.I. No. 16230. Eastman Kodak Co., Rochester, NY. (11) J. Steinhardt and E. Zaiser, Combination of Wool Protein with Hydroxyl IonsJ. Biol. Chem., 183, 780-802 (1950).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)