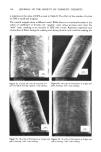
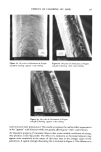
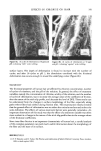
242 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (2) K. W. Wolf, "An Experimental Study of Interfiber Friction in Surgical Sutures by the Twist Method," Master's Thesis, North Carolina State University (1979). (3) D.C. Prevorsek, R. H. Butler, Y. D. Kwon, G. E. R. Lamb, and R. K. Sharma, Influence of fibre properties on wrinkling behavior of fabrics VII. Effects of morphology on fibre properties, Text. Res. J., 47, 107-126 (1977). (4) Handbook of Chemistry and Physics, 55th ed., R. C. Weast, Ed., (CRC Press, Cleveland, 1974), p D120. (5) J. Lindberg and N. Gralen, Measurement of friction between single fibers II. Frictional properties of wool fibers measured by the fiber-twist method, Text. Res. J, 18, 287-301 (1948). (6) B. S. Gupta, Unpublished Research Work. (7) J. H. Bradbury, The theory of shrinkproofing wool part II: Chemical modification of the fiber surface and its effect on felting shrinkage, friction and microscopic appearance, Text. Res. J., 31,735-743 (1961). (8) K. R. Makinson, Some new observations on the effects of mild shrinkproofing treatments on wool fibers, Text. Res. J, 38, 831-841 (1968). (9) K. R. Makinson, Mechanisms involved in shrinkproofing by degradative treatments, AppL Po]ym. Sym., #18, 1083-1096 (1971). (10) K. R. Makinson and I. C. Watt, Some physical effects of acid wet chlorination on wool fibers in relation to its use as a shrinkproofing treatment for wool, Text. Res. J, 42,698-703 (1972). (11) K. R. Makinson, The role of chlorine in oxidative antifelting treatments of wool, Text. Res. J, 44, 856-857 (1974). (12) F. Alexander and D. Gough, The reaction of oxidizing agents with wool 4. The reactivity of tyrosine, Biochem. J., 48, 504-511 (1951). (13) H. Zimmerman, Felt resistant wool by wet chlorination, Am. DyestuffReptr., 36, 473-476 (1947). (14) L. Shapiro, Shrinkage control of wool by wet chlorination, Am. DyestuffRptr., 37, 376-380 (1948). (15) P. Alexander, R. F. Hudson, and M. Fox, The reaction of oxidizing agents with wool I. The division of cystine into two fractions of widely differing reactivities, Biochem. J., 46, 27-32 (1950). (16) P. Alexander, D. Gough, and R. F. Hudson, The reaction of oxidizing agents with wool 3. The influence of the morphology on the rate of reaction, Biochem. J, 48, 20-27 (1951). (17) P. Alexander, M. Fox, and R. F. Hudson, The reaction of oxidizing agents with wool 5. The oxidation products of the disulphide bond and the formation of a sulphonamide in the peptide chain, Blochem. J., 49, 129-138 (1951). (18) M. Harris and D. Frishman, Some aspects of the chlorination of wool to produce shrink resistance, Am. DyestuffReptr., 37, 52-55 (1948). (19) G. Valk, Reaction between chlorine and wool proteins, Proc. 3rd Int. IVool Text. Res. Conf, Paris, 2, 371-381 (1965). (20) A. Kantouch and S. H. A. Fattah, Oxidation of wool with chlorine and some chlorinated compounds, App/. Po/ym. Symp. #18, 317-323 (1971).
j. Soc. Cosmet. Chem., 33, 243-248 (August 1982) The stability of 2-pyridinethiol-l-oxide, sodium salt, as a function of pH ROBERTJ. FENN and DAVID A. CSEJKA, Olin Research Center, P.O. Box 30-275, New Haven, CT06511. Received December 9, 1981. Synopsis The hydrolysis of sodium-2-pyridinethiol-l-oxide has been studied in aqueous solutions buffered at pH 4.1, 7.1, and 10.0 at 5øC and 40øC. The sample concentration studied was approximately 100 ppm. Samples stored at 5øC showed very little loss of starting material over the 30 day duration of the study. Samples held at 40øC showed significant loss only at pH 10.0. The major product found at pH 4.1 and 7.1 was the disulfide oxidation product, while at pH 10.0 the major product was the sulfinate salt, formed by attack of hydroxide ion on the disulfide. Additional 30-day experiments run at pH 7, 8, 9, and 10 indicated a gradual shift of the product distribution from the disulfide to the sulfinate with increasing pH. The sulfinate salt was shown to be the end product of alkaline hydrolysis up to 30 days by its demonstrated stability at pH 10 and 40øC. An authentic sample of 2-pyridinesulfinic acid-l-oxide was prepared and characterized as an aid to product identification and quantitation. INTRODUCTION The sodium salt of 2-pyridinethiol-l-oxide (NaPT) is used as a preservative in some cosmetic formulations at typical use levels of 100 to 1000 ppm. Information was desired concerning the stability and possible degradation products of this compound in formulations at various pH values. Accordingly, studies were undertaken to monitor stability of aqueous buffer solutions of 100 and 500 ppm NaPT from pH 4 to 10 at 5 ø and 40øC. Degradation products were identified and quantitated during the course of the 30-day study. Based upon the results of the present study, recommendations can be made concerning the product pH range suitable for the applications of NaPT as a preservative. EXPERIMENTAL APPARATUS Polarograms were obtained using a Princeton Applied Research Model 174 Polaro- graphic Analyzer equipped with either a Model 172A Drop Timer or a Model 303 Static 243
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)