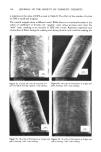
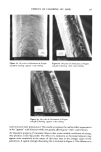
230 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS F /x- N , (1) in which/a is the coefficient of friction, F the frictional force, and N the normal load. It has been shown by Bowden and Tabor (1) that Equation 1 applies only to materials which deform plastically, such as metals, plastics, etc. Materials deforming elastically or viscoelastically exhibit a departure from this behavior. Thus, in fibers, which are generally viscoelastic, the coefficient of friction is a function of factors such as fiber dimensions, physical properties, testing environment, and the testing conditions (2). It is for this reason that the results of fiber friction tests have generally shown a lack of reproducibility. In research requiring reproducible measurements, therefore, it is essential that tests be carried out under strictly controlled conditions, with as many variables as possible maintained constant. When force is applied to the sliding member, energy is stored in the elastic parts of the system. Sliding begins, and when this force exceeds the value necessary to maintain a constant speed the fiber accelerates, overshoots, then decelerates. This process keeps repeating, producing the stick-slip effect (3). Thus, one can obtain values of static (Fs) and kinetic (Fk) frictional forces from a frictional trace and from Equation 1, the corresponding values of the static (/as) and the kinetic (/Xk) coefficients of friction. The nature of the stick-slip also seems to depend upon the viscoelastic nature of the fiber and the method of testing. For a given speed of sliding, relatively soft surfaces tend to give a more pronounced stick-slip trace, hard surfaces a straighter trace. One of the unique features of hair and other animal fibers is that they possess a cuticular surface with scales. The consequence of this is that the fiber displays a directional character in frictional measurements. The value of the coefficient of friction of human hair obtained when rubbing against the scales (/xa) can be significantly higher than that obtained when rubbing with the scales (/Xw). The difference bewteen the two values "/xa -/Xw," is called the differential frictional effect, or DFE. The magnitude of DFE relates strongly to the tendency of the fibers to felt, or compact into a dense mass. Any treatment which tends to destroy and/or soften the scales lowers the value of DFE. Thus, the effect of a treatment on the fiber behavior can lead to insights on the changes in surface of the fibers. Since hair performance in such contexts as manageability, style holding, and ease of combing is more or less surface related, the changes in friction and surface character with a treatment could be expected to be informative as to such hair assembly behavior. NATURE OF CHLORINE IN AQUEOUS SOLUTIONS The composition of a chlorine solution in respect to the active oxidizing species present is determined by its hydrogen ion concentration. Depending on the pH, the chlorine is present either as C12, HOC1 and OCI-, or as mixtures of these (Figure 1). The oxidizing potential of a chlorine solution and, consequently, the nature and the magnitude of its effect on hair keratin, can be expected to depend greatly on its pH (Table I). It might be noted that the concentration of chlorine in solution can change due to reaction and to changes in solution pH. This warrants frequent checks and, if necessary, corrections in order to assure accuracy in experimental studies.
EFFECTS OF CHLORINE ON HAIR 231 IOO 9o 8o • 7o o 60 n,. 50 z "• 40 z o • 3o 2o IO Cl2 HOCJ OCl- i/ •'•' •'• ' • '"""*•' /•.,,•'"" • • • / \. / i x. / i / \\ /I \•\ I II ? \ / /' \ / .... ß /', , , •-..--"" ,/ , , ' ,%-...._ _ •, I 2 3 4 5 6 7 8 9 IO pH Figure 1. Composition of a sodium hypochlorite solution as a function of pH. EXPERIMENTAL Materials and Chlorination Procedure Dark brown Caucasian hair obtained from the De Meo Brothers Company was used in this study. The hair was extracted in a 50/50 chloroform/methanol mixture and tied into 2.0 g tresses. For all tests, the chlorine solution was prepared by dilution of a sodium hypochlorite solution. The chlorine concentration was analyzed by iodometric analysis with KI and Na2S20 3 before and after each cycle. The pH of the solution was varied and controlled with HC1. The treatments were carried out at room temperature using 500 ml liquor: 1 g hair ratio. During the soaking period, the chlorine solution was covered with a polyethylene wrap. Two sets of experiments were performed. In one, the pH level of the solution was maintained constant at 8.0, and the tresses were treated with 20, 40, and 60 cycles of Table I Standard Oxidation Potentials for Reactions of Chlorine Compounds with H20 [4] Solution Reaction E* (volts) Acid HOC1 + H + + 2e-• CI- + tt20 --1.49 Cl 2 q- 2e- --} 2Cl -1.36 Base Clo-- + H20 + 2e'- • C1 + 2OH -0.90
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)