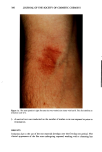
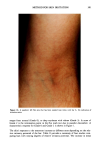
266 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS antibacterial. Consequently, we wished to determine if the agar patch test could detect the skin substantivity and antibacterial activity of triclosan-containing liquid soaps. An additional purpose was to compare the results from the agar patch test on this soap with those from the finger imprint test (4). This test, like the agar patch test, was developed to evaluate antibacterial soap bars by measuring the bacteriostatic activity deposited on panelists' skin. In contrast to the agar patch test, triclocarban is poorly detected in the finger imprint test (4). Liquid soaps or triclosan-containing products have not been previously evaluated by either test method. EXPERIMENTAL SOAPS 1. Liquid soap A (antibacterial, commercial product) contained water, ammonium lauryl sulfate, lauramide DEA, sodium laureth sulfate, disodium ricinoleamido MEA-sulfosuccinate, citric acid, DMDM hydantoin, tetrasodium EDTA, FD&C yellow #5, and FD&C red #4. The active ingredient was triclosan. 2. Liquid soap B (nonantibacterial, made in the Dial Technical Center pilot plant) was the same formula as liquid soap A except that it contained no triclosan. 3. Liquid soap C (nonantibacterial, commercial product) contained water, lauric acid, oleic acid, potassium hydroxide, glycerin, fatty acids, preservative, and fragrance. 4. Soap bar D (nonantibacterial, commercial product) contained sodium tallowate, so- dium cocoate, potassium tallowate, potassium cocoate, sodium chloride, alcohol insolubles (inorganic material), glycerin, magnesium silicate, chelating agent, and fragrance. 5. Soap bar E (nonantibacterial, made in Dial Technical Center pilot plant) contained sodium tallowate, sodium cocoate, water, sodium chloride, glycerin, preservative, and color. PANELISTS Informed consent was obtained from all panelists. Only volunteers with undamaged skin were studied. The panelists participated in a one-week washout (handwashing and bathing) with bar soap E (placebo) prior to beginning the study. They were instructed to refrain from using cosmetic products such as lotions or perfumes on the hands or forearms for 24 hours prior to the test. They were also told not to use antibacterial antiperspirants, deodorants, soaps, or shampoos. Nonpowdered rubber gloves were pro- vided for panelists to use when hands came in contact with acids, bases, or solvents. Panelists were required to refrain from swimming in biocide-treated pools, hot tubs, spas, etc., for 24 hours prior to the test. Panelists with open cuts or wounds on their hands or forearms on the day of the test were excluded from participating in the study. At the conclusion of each test, the panelists washed their hands with antibacterial liquid soap A to remove any bacteria that might have been picked up during the test. AGAR PATCH TEST Test culture. A streptomycin-resistant derivative of Staphylococcus epidermidis ATCC
ANTIMICROBIAL TESTING OF SOAPS 267 14990 was maintained on trypticase soy agar (BBL) plates containing 1 mg/ml of strep- tomycin sulfate. The third consecutive overnight culture of the streptomycin-resistant S. epidermidis grown in tryptic soy broth (Difco) and diluted with 0.1% peptone was used in this test. Falcon 1008 petri dishes (35 ( 10 mm) containing 11 ml of trypti- case soy agar with 1 mg/ml streptomycin sulfate were streaked with 1000-2000 CFU/ plate using a 4-mm loop. The streptomycin in the media prevents contamination of the plates by resident skin bacteria during the test procedure (1). Wash procedure. The hands and forearms were wetted under warm, running tap water. The hands were washed with placebo soap bar E to remove transient bacteria. In the first test, one pump of liquid soap B (nonantibacterial) was added to one hand and carefully rubbed over the volar surface of the opposite forearm for 15 seconds. From the tap, a small volume of water was added to the same hand, and the volar surface of the opposite forearm was lathered for 30 seconds. The hand and forearm were rinsed for 30 seconds and blotted dry with paper towels. The identical procedure was repeated with liquid soap A (antibacterial) using the other hand and forearm. The assignment of the soaps to panelists' hands and forearms followed a computer-generated randomization table. In the second test, commercial liquid soap C (soap-based) was used instead of pilot plant-made liquid soap B (detergent-based). Also, eight panelists were used in the second test instead of the four used in the first test. Otherwise, the two tests were conducted identically. Test procedure. Three S. epidermidis streaked trypticase soy agar plates ("agar patches") containing 1 mg/ml streptomycin sulfate were attached to the volar surface of each forearm with 1-inch ( 3-inch strips of Microfoam © tape (3M) and wrapped with an elastic bandage (Becton-Dickinson) for 30 minutes. The plates were then removed from the forearms, placed in 150 ( 25-mm petri dishes, and incubated at 35øC. Streaked controls, which were not attached to the forearms, were incubated in the same way. Twenty-four hours later, the colonies in all plates were counted with the aid of a 10X dissecting microscope. FINGER IMPRINT TEST Test cultures. Staphylococcus epidermidis ATCC 14990 and Escherichia coli ATCC 11229 were individually grown in brain heart infusion broth (BBL), and a 1% inoculum of each bacterium was prepared in individual bottles of melted and cooled (48øC) trypticase soy agar. Bacteria-seeded plates were prepared by placing 15 ml of trypticase soy agar in sterile petri dishes and allowing the agar to harden. Then 5 ml of the 1% culture in the melted trypticase soy agar was carefully spread over the surface of the hardened trypti- case soy agar and allowed to solidify. Wash procedure. Panelists first washed their hands with a placebo soap bar (E) to remove transient bacteria. In the first test, two pumps (3.7 g) of either liquid soap A or B were delivered into panelists' wet hands from a dispenser. The panelists then applied the soap by rubbing it over their hands for 15 seconds. Panelists then wetted their hands with a small amount of tap water and lathered the soap for 60 seconds. Finally the panelists rinsed their hands under the tap for 30 seconds and blotted both hands dry with paper towels.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)