WATER RETENTION AND PSEUDOCERAMIDES 275 N,N-bis(3-hexadecyloxy-2-hydroxypropyl)-2-aminoethanol, {3}: Sodium N,N-bis(3- hexadecyloxy-2-hydroxypropyl)glycinate, {4}: N,N-bis(3-hexadecyloxy-2-hydroxy- propyl)glycinate, {5}: N,N-bis(3-hexadecyloxy-2-hydroxypropyl)acetamide, {6}: 3,4- di-O-(z)-9-octadecenoyl-D-mannitol, {7}: N-hexadecylhexadecanamide, {8}: N-(3-hex- adecyloxy-2-hydroxypropyl)-N-methylhexadecanamide, {9}: N-2-hydroxy- eicosyl-N-2-hydroxyethylhexadecanamide, {10}: N-(3-hexadecyloxy-2-hydroxy- propyl)-N- 3-hydroxypropylhexadecanamide, { 11 }: N-(3- hexadecyloxy- 2-hydroxy- propyl)-N-6-hydroxyhexylhexadecanamide, {12}: N-(3-hexadecyloxy-2-hydroxy- propyl)-N-2-(2-hydroxyethoxy)ethylhexadecanamide, {13}: N-(3-hexadecyloxy-2-hy- droxypropyl)-N-2,3-dihydroxypropylhexadecanamide, {14}: N-2-hydroxyethyl-N- (2-hydroxy-3-tetradecyloxylpropyl)octadecanamide, {15}: N-(3-dodecyloxyl-2-hydroxy- propyl)-N-2-hydroxyethyloctadecanamide, {16}: N-(3-hexadecyloxyl-2-hydroxy- propyl)-N-2-hydroxyethyloctadecanamide, {•7}: N-2-hydroxyethyl-N-(2-hydroxy-3- octadecyloxylpropyl)octadecanamide, {18}: N-2-hydroxyethyl-N-(2-hydroxy-3-octade- cyloxypropyl)decanamide, {19}: N-2-hydroxyethyl-N-(2-hydroxy-3- tetradecyloxypropyl)dodecanamide, {20}: N-(3-hexadecyloxy-2-hydroxypropyl)-N-2- hydroxyethyldodecanamide, {21 }: N- 2-hydroxyethyl-N-(2-hydroxy- 3-octadecyloxy- propyl)dodecanamide, {22}: N-2-hydroxyethyl-N-(2-hydroxy-3-octadecyloxy- propyl)hexadecanamide, {23}: N-(3-hexadecyloxy-2-hydroxypropyl)-N-2-hydroxyethyl- tetradecanamide, {24}: N-(3-docosyloxy-2-hydroxypropyl)-N-2-hydroxyethyldecana- mide, {25}: N-(3-decyloxy-2-hydroxypropyl)-N-hydroxyethyldecanamide, {26}: N-(3- decyloxyl-2-hydroxypropyl)-N-2-hydroxyethyloctadecanamide, {27}: N-2-hydroxy- ethyl-N-(2-hydroxy-3-tetradecyloxypropyl)tetradecanamide, {28}: N-(3-hexadecyloxy- 2-hydroxypropyl)-N-2-hydroxyethyl-methylheptadecanamide, {29}: N-2-hydroxy- ethyl-N-(2-hydroxyl- 3-methylheptadecyloxypropyl)methylheptadecanamide, {30}: N- 2-hydroxyethyl-N-(2-hydroxy-3-methylheptadecyloxypropyl)hexadecanamide,{31}: N-(3-decyloxyl-2-hydroxypropyl)-N-2-hydroxyethyldocosanamide, {32}: N-2-hydroxy- ethyl-N-(2-hydroxy-3-octadecyloxypropyl)tetradecanamide, {33}: N-(3-dodecyloxy-2- hydroxypropyl)-N-2-hydroxyethyl-(z)-9-octadecenamide, {34}: N-2-hydroxyethyl-N- (2-hydroxy-3-tetradecyloxylpropyl)hexadecanamide, {35}: N-(3-dodecyloxy-2-hydroxy- propyl)-N-2-hydroxyethylhexadecanamide, {36}: N-2-hydroxyethyl-N-(2-hydroxy-3- tetradecyloxypropyl)decanamide, {37}: N-2-hydroxyethyl-N-(2-hydroxy-3-tetradecyloxyl- propyl)docosanamide, {38}: N-2-hydroxyethyl-N-(2-hydroxy-3-tetradecyl(oxypropyl)- (z)-9-octadecenamide. Petrolatum, squalene, octyl-dodecyl myristate, cholesterol, cholesterol ester, and stearic acid were obtained from Wako Chemical (Tokyo, Japan). Naturally occurring ceramide was purchased from Sigma Chemical Co. (Saint Louis, MO). TREATMENT WITH ACETONE/ETHER OR SODIUM DODECYL SULFATE The forearms of 7- 13 healthy volunteers, aged 24-33 years, were used for each experi- ment. Open-end, 3-cm-diameter cylinders filled with 10 ml of acetone/ether (1/1) or sodium dodecyl sulfate (SDS) at 5% concentration were gently pressed with occasional shaking onto the sample areas for 30-min periods to induce dry skin (day -1). They induced a chapped and scaly appearance of the stratum corneum that persisted until at least day 4 after treatment (2).
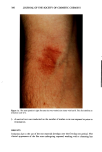
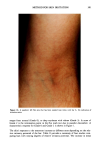
276 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS APPLICATION OF COMPOUNDS Synthetic compounds were solubilized at 1-5% concentrations in squalene containing 1% o•-monomethyl heptadecyl glyceryl ether (GE, Kao Corp, Tokyo, Japan) or W/O cream consisting of 2% GE, 3% petrolatum, 5% squalane, and 10% octyl-dodecyl myristate. This cream was applied daily at 0.014 ml/area (approximately 2 p•l/cm 2) from the first day (day 0) after 30 min acetone/ether or SDS treatment for 2-3 succes- sive days, or once one hour after 30-min acetone/ether treatment. MEASUREMENT OF WATER-RETAINING CAPACITY OF THE STRATUM CORNEUM Water-retaining capacity of the stratum corneum was measured according to the method of Tagami et al. (8). Changes in water content of the treated areas were mea- sured by a capacitance conductance meter (model IB-354, IBS Inc., Japan). Treated areas were rinsed with water at 37øC and then, after keeping volunteers at 20øC and 50% humidity for 20 min, were measured for skin reaction and conductance value 24 hours (day 3) or four hours after the last sample application, or daily through the period of experiment. Conductance measurements were carried out five times at the same area, and the values were averaged to obtain individual values. MEASUREMENT OF SKIN REACTION The skin reaction, including scaling, was observed three days (day 3) after acetone/ether treatment under the same conditions as conductance measurement. Scaling was assessed according to the following scale: no scaling = 0, slight scaling = 1, moderate scaling = 2, marked scaling = 3. STATISTICS The level of significance of the difference was calculated by Student's t-test for paired comparison for conductance values and by Friedman's test for scaling. RESULTS Topical applications to acetone/ether-induced dry skin of the synthetic pseudoceramides that have different polar groups but the same alkyl chain length with an ether bond, emulsified at 1% in a W/O cream, showed a significant recovery of the decreased con- ductance value, as compared with nontreatment control or base cream when the polar group has an amide bond, while the application of base cream only failed to produce any significant recovery (Figure 1). The applications of naturally occurring ceramide from calf brain (NOC) also showed a significant recovery as compared with the nontreatment control (Figure 2). Among three different structures with amide bonds in the main linkage, a structure with two hydroxyl groups {1} is found to exhibit the highest po- tential to improve water-retaining properties, accompanied by a significant improve- ment of scaling induced by acetone/ether treatment (Figure 3). Comparing compound {9} with compound {1} in Figure 4, it is clear that the substitution of a carbon-carbon bond for the ether bond found near the polar end of alkyl chain provides a less efficient recovery effect. This suggests that the ether bond in this structure (B) is similar to a
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)