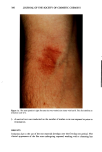
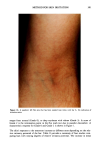
J. Soc. Cosmet. Chem., 40, 273-285 (September/October 1989) Water-retaining function in the stratum corneum and its recovery properties by synthetic pseudoceramides GENJI IMOKAWA, SHUICHI AKASAKI, AKIRA KAWAMATA, SHINJI YANO, and NAOTAKE TAKAISHI, Tochigi Research Laboratories, Kao Corporation, Tochigi, Japan. Received January 18, 1989. Synopsis We have recently found that lipids that form lameliar structures in the intercellular spaces of the stratum corneum can be specific modulators for water-retaining properties of the stratum corneum. Among the intercellular lipids, ceramide (A) fractions exhibit the highest capacity for recovering diminished water-re- taining properties. In order to clarify the role of ceramides in the water-retaining function of the stratum corneum, we have synthesized pseudoceramide (B) derivatives and examined their potential for repairing the lipid-depleted stratum corneum, in which a marked decrease in the water-retaining properties is found. Synthesized pseudoceramide derivatives are characterized by structures having both amide or nitrogen bonds and hydroxyl groups as hydrophilic units, as well as two long alkyl chains. When the polar group has an amide bond in the main linkage with hydroxyl ether binding at nitrogen atom, topical applications of these compounds (solubilized at 1-3% in squalane or W/O cream) to acetone/ether or sodium dodecyl sulfate-induced dry skin showed a significant recovery of water-retaining properties--accompanied by an improvement in scaling--over that induced by base cream. Analysis of alkyl chain properties has revealed that a structural requirement for the recovery of the water-retaining capacity is the presence of saturated- straight alkyl chains whose structural characteristics are very similar to naturally occurring ceramide in the stratum corneum and the absence of unsaturation or methyl branching. However, the observed alkyl chain length (14-18 carbons) preferred for water-retaining function is different from that of the major naturally occurring ceramides, indicating differential contributions of ceramide structures to stratum corneum func- tions. The present evidence suggests that ceramides with relatively shorter alkyl chain lengths serve as water modulators in the multilipid bilayers within the stratum corneum. OH H OH CH 2 CH3(CH2)12C = C - CH - CH - NH - CO(CH2)22CH3 Ceramide N-Acyl Sphingosine (A) 273
274 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS OH CH 2 OH CH 2 I R - O - CH 2 - CH - CH 2 - N - CO - R A-Pseudoceramide (B) INTRODUCTION We have recently found that lipids that form lameliar structures in the intercellular spaces of the stratum corneum can be specific modulators for water-retaining properties of the stratum corneum, whose function is reversible by topical applications of the intercellular lipids (1,2). Among the intercellular lipids, the ceramide fraction is found to possess the highest capacity for repairing diminished water-retaining properties (3). Ceramides are unique heterogeneous materials capable of forming multilamellar struc- tures in the absence of phospholipids. This lameliar-forming ability is suggested to be involved with the water-retaining properties (3,4), as was previously elucidated for the water barrier function of the stratum corneum (5). Although several species of cera- mides have been identified from the intercellular lipids of the stratum corneum (6), little is known about the precise potential for their proposed functions because of lim- ited availability of natural ceramides for experimentation. Lipid-depleted stratum cor- neum following acetone/ether (3) or sodium dodecyl sulfate treatments (7) was found to be useful as a model for a deficiency in the amount of ceramides that is directly involved in the induction of dry skin, because ceramides are major and essential comRonents of stratum corneum lipids. In order to clarify the role of ceramides in the water-retaining function of the stratum corneum and the structural characteristics responsible for main- taining water, we have synthesized pseudoceramide derivatives and examined their re- pair potential on the lipid-depleted stratum corneum, in which a marked decrease in the water-retaining properties is found. In this paper, we report structural features of ceramides relevant to the water-retaining function. MATERIALS AND METHODS MATERIALS Amide derivatives were principally synthesized by the following stepwise process: Gly- cidylether was first produced by reaction of the appropriate alcohol, epichlorohydrin, and 50% aqueous tetrabutylammonium bromide in hexane with 50% aqueous sodium hydroxide at 50-55øC. The appropriate glycidylether was then added dropwise under heating at 60-70øC to the ethanolamide and ethanol, to yield an ethanolamide adduct (B) after purification by flash chromatography. In some cases, the ethanolamide adduct was stirred with methylcarboxylate and powdered potassium hydroxide under reduced pressure at 80-100øC to yield other amide derivatives that were double recrystalliza- tion from ethanol. The synthesized amide derivatives are as follows: {1}: N-(3-hexadecyloxy-2-hydroxypropyl)-N-2-hydroxyethylhexadecanamide, {2}:
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)