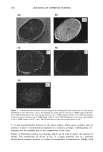
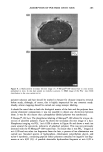
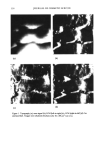
338 JOURNAL OF COSMETIC SCIENCE NOVEL BOTANICAL SKIN ANTi-IRRITANTS DISCOVERED BY HET-CAM ASSAY Danielle Simonot, Tracy Wilson and Warren Steck Fytochem Products Inc., 110 Research Drive, Saskatoon, SK S7N 3R3 Canada Introduction to the HET-CAM (HeWs egg test - chorioailantoic membrane) The HET-CAM has bccn used as a toxicological and pharmacological model for many years 4 and has bccn established and proven to be a robust test with good predictive value of irritation potential •. In the progressing scientific movement to replace animal testing in lieu of more humane techniques, the HET- CAM allows for ethical, non-animal screening of irritating compounds and has become a common and validated technique. While the HET-CAM has traditionally bccn utilized to measure the irritating potential of substances, its methods have bccn modified to assess the anti-irritancy activity of compounds as well. The HET-CAM involves the observation of vascular injury to the extra-embryonic chorioallantoic membrane (CAM) of 10-day old fertilized hcn's eggs upon application of a substance of study. Eggs up to 10 days of age are still largely considered foodstuff' therefore their utilization does not conflict with legal or ethical issues, particularly animal protection laws. Further, while the CAM is a complete and living tissue, it lacks sensory inncrvation 3 therefore is regarded as insensitive to pain 4 and suitable for the testing of irritants. The HET-CAM incorporates aspects of both ir vitro and in vivo test systems. Its in vivo component involves the utilization of a living, highly vascularized and metabolically-active membrane which can thereby provide an accurate depiction of the test substance on living tissue. However, it also provides several advantages of in vitro test methods including simplicity, rapidity, sensitivity, case of performance and relative cheapness 5. Based on the technique described by Daunhardt ctal. (1996) 4, testing is also being accomplished by injection of test substances directly into the egg albumen. This permits prolonged incubation of samples for up to six hours, a feat not possible with the traditional HET-CAM procedure which exposes the CAM membrane upon application of the test substance and thus restricts the amount of time the membrane may remain exposed and unperturbed. This method also allows the testing of substances which may not bc normally absorbed into the CAM. Modifying the HET-CAM to assess anti-irritancy Anti-irritant activity of an aqueous plant extract can be measured by the delay of the typical landmark irritation phenomena following the application of a standard irritant: hemorrhage, lysis and coagulation. This method has permitted the screening and identification of aqueous plant extracts which possess anti- irritating properties. Skin patch testing has confirmed this activity in at least one plant extract identified by this assay thus far. Materials & Methods Fresh, fertile, White Leghorn hen's eggs are incubated for a period of ten days at a temperature of 37øC with periodic rotation. Eggs are placed with the blunt end up to correctly position the airsac above the CAM. On the l0 th day of incubation, the eggs are candled and their airsac delineated by pencil. Infertile eggs or those with non-viable embryos, clearly distinguishable by the lack of embryo movement or abnormalities in blood vessel development, are discarded by freezing. All eggs are then weighed and only those between 50-60 g utilized in order to maintain a standard and uniform testing criteria. Testing is carried out in a sterile, horizontal flow hood and by methodology described by Spielmann 5. To expose the CAM for testing, the eggshell is cut away around the previously delineated line by a Dremel tool with a small cutting disk attached to the flexible head. The eggshell cap is removed by tissue forceps and the visible outer shell membrane is moistened with 0.9% isotonic NaC1 solution and incubated for five minutes to soften the membrane. The NaC1 solution, along with all samples and standard irritants to be utilized in the assay, are kept heated to physiological temperature in a water bath. Once the NaC1 solution is wicked off and the shell membrane is carefully removed by blunt forceps, the exposed CAM is examined for any damage or abnormalities, either of which will result in its ejection from the assay. Aqueous plant extract is carefully applied to the CAM by pipette which is then covered by plastic wrapping to keep the membrane moist, and the egg is placed in the incubator for a period of 20 minutes. Each plant extract is tested by three replicates. Following incubation, 15% lactic acid (LA), the standard
2000 ANNUAL SCIENTIFIC SEMINAR 339 irritant, is applied to the CAM. The time taken for the three irritation phenoma (hemorrhage, lysis and coagulation) to occur up to a maximum of 5 minutes is then recorded. Following this observation, the egg is destroyed by freezing. Prior to each assay trial, two eggs are also tested with standard irritant (15% LA or 1% SDS) to provide a basal measure of standard irritation. Measuring The Irritation Phenomena Irritation of the CAM is gauged by the appearance of three landmark irritation phenomena (as described by Steilingl): hemorrhage (H), observed as bleeding out from blood vessels lysis (L), indicated by the disappearance of small blood vessels on the CAM as a consequence either of bleeding, dystonia of these fine vessels or disintegration and coagulation (C), either intravascular (thrombosis) or extravascular which tends to increase the CAM opacity. The irritation score (IS) of a substance, described by Spellmann 5, is then determined by the following formula: IS: [(301-H)/30015 + [(301-L)/30017 + [(301-C)/30019 The application of a standard irritant determines the basal time required for these phenozaa to occur. Following the application of a plant extract to the CAM, anti-irritancy can be determined by the ability to delay the occurrence of these phenoma following application of the standard irritant. Results & Discussion Of a multitude of plant extracts tested, a select few were identified which showed anti-irritant properties illustrated by a significant reduction in the IS score and confirmed by human skin patch testing. Two of these plants are from the same botanical family. Subsequent isolation and testing of the plant active showed an even further reduction of irritation phenomena. Additionally, it was found that by utilization of the injection method, the longer the incubation time, the increased the effect. At present, the Draize rabbit eye test is the only method accepted worldwide for registration of new chemicals into the marketplace I. Alternatives to this method have actively been sought out for the last few decades and one of the most successful assays evaluated so far has been the HET-CAM, the hews egg-test on the chorioallantoic membrane of fresh, fertilized eggs. The HET-CAM has provided a convenient, reliable, mechanism-independent screening assay for anti-irritant action of natural substances. Hydrocortisonc Plant extracZ HET-CAM T•ting Plant Extract (Solntlon and Lotion) Reduce• UV-induced Erythema 25 ll t 20 mummmamma 0 o i t 3 4 s ß ? ist Sirs of Ir•jtatioa I•ødy Factor (Sample Deh.vlCoatrol Delay) 4 24 Hours After Treatment • n Ut%l•m •r ufk. t EI•,I• IoU• Plant Extract Reduces Chemically Induced Erythema 13 Control ß Plant extract 50 :• 20 .• •o o 0.5 I 4 24 Hours After Treatment An aqueous plant extract with promising anti-irritant activity, as identified by the HET-CAM, and 1% hydrocortisone were tested competitively in a 24-hour human patch test employing 15% lactic acid as irritant. In this test the extract gave better short-term remediation of the symptoms or symptoms than was obtained with the steroid. During another human skin test, the extract was also found to reduce the effects ofUV-induced erythema. Therefore, the HET-CAM has been shown to have potential as a screening tool for anti-irritant plant extracts. 1 - Steiling W., Braeher M., Courtellemont P. & de Silva O. 1999. Toxicology in Vitro 13: 375-384. 2 - BagIcy D.M., Waters D. & Kong B.M. 1994. Fd Chem. k Toxic. 32(12): 1155-1160. 3 - Leighton J., Nassauer J. & Tchao R. 1985. Fd Chem. Toxic. 23(2): 293-298. 4 - Dannhardt G., Kreher M., Nowe U. & Pies A. 1996. Arch. Pharm. Pharm. Med. Chem. 329:301-310. 5 - Spielmann H. 1995. In: O'Hare S. & Atterwill C.K. (eds) Methods in Molecular Biology Vol.43: In Vitro Toxicity Testing Protocols. Humana Press Inc., Totowa NJ
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)