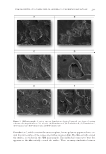
JOURNAL OF COSMETIC SCIENCE 176 Solar elastosis is considered to be a sign of chronic sun exposure–related ageing, which is histologically characterized by reduced numbers of dermal collagen fi bers and the accumu- lation of dystrophic elastotic material (4). The elastin fi bers attach themselves forming elastotic aggregates, which form abnormal nonfunctional disintegrated elastic fi bers, leading to a premature ageing phenotype, associated with loss of elasticity, yellow skin, and deeper wrinkles. With ageing, elastic fi bers are abundant, thickened, and disorganized. Elastic fi bers are formed mainly during embryonic development with the deposition of tropoelastin, the soluble precursor of elastin, on a scaffold of fi brillin rich in microfi brils (5–7). The lysyl oxidase (LOXL1) catalyzes the formation of covalent cross-links between some lysine residues of two adjacent tropoelastin molecules, which become insoluble polymer elastin. Mature elastic fi bers consist of an external coat of microfi brils and an amorphous core of cross-linked elastin. These cross-linked amino acids consolidate the polymer, procure its elastic function, and ensure its resistance. The cross-linking step is essential to strengthen the growing elastic fi bers, and this highlights the important role played by LOXL1 in elastogenesis. Moreover, elafi n, also called skin-derived antileukoproteinase, is a serine protease inhibi- tor, mostly produced by epithelial cells. In the skin, keratinocytes are the main source of this molecule. Although elafi n is not detectable in normal skin, it is secreted abundantly in psoriasis and other infl ammatory skin disorders (8). Elafi n acts in various ways on the cutaneous immune homeostasis by not only exerting antiprotease but also by causing immunomodulatory and antiproliferative effects. In the actinic elastosis of sun-damaged skin, fi broblasts express elafi n, (also known as peptidase inhibitor), which binds to elastin. The elafi n–elastin complex hampers the elastolytic regular process, leading to the accu- mulation of abnormal disintegrated elastic fi bers and aggregates (9). The accumulation of elastotic material can be caused by multiple factors, such as a decrease in collagen, an increase in the synthesis of elastin and/or a breakdown of existing and nascent elastic fi bers. We proposed in this study to rebalance the key partners involved in the solar elastosis mecha- nism to improve the level of functional elastic fi bers in the skin exposed to sun radiation. We tested the effect of our new specifi c Hamamelis extract on LOXL1 expression. By increasing LOXL1 expression, our specifi c Hamamelis extract balances elastin/LOXL1 expressions for getting more functional elastin fi bers. The potential of our specifi c Hamamelis extract to counteract the accumulation of abnor- mal elastin fi bers was also evaluated by measuring its capacity to inhibit elafi n expression. Thanks to this clinical study, we have shown that our specifi c Hamamelis extract main- tains youthful skin when exposed to the sun and may be used as a treatment applied at night to relieve the harmful effect of the sun light during the day. METHODS IN VITRO BIOLOGICAL TESTS Active ingredient. In vitro: Hamamelis extract is an extract of leaves of Hamamelis virginiana. Clinical trial. Hamamelis is a formulation containing 1% of water, pentylene glycol, Hamamelis virginiana (witch hazel) leaf extract, xanthan gum, and caprylyl glycol.
UNDERSTANDING SOLAR SKIN ELASTOSIS 177 REAL-TIME PCR FOR ANALYSIS OF ELASTIN AND LOXL1 EXPRESSION LEVELS Cell culture. Fibroblasts (63-year-old donor) were cultured until confl uence. They were then exposed to ultraviolet A (UVA) (7.5 J/cm2) and cultured for 24 h. LOXL1 and elas- tin expression was evaluated by quantitative - reverse transcription - polymerase chain reaction (q-RT-PCR). In the second experiment, fi broblasts (63-year-old donor) were cultured until confl uence. Three different conditions have been used. The control culture was maintained for 24 h and then exposed to UVA (7.5 J/cm2). The culture was maintained for 16 h. In the post irradiation condition, the Hamamelis extract was added just after the irradiation, and maintained for 16 h. In the pre- and postirradiation conditions, the specifi c Hamamelis extract was added in the culture at confl uence and for 24 h. Then the culture was exposed to UVA (7.5 J/cm2) and the Hamamelis extract was added for additional 16 h. Assay method. The cells were washed with phosphate buffer solution (PBS) and then total RNA was extracted (Spin Vacuum Total RNA Isolation System Z3500, Promega, Char- bonniere les bains, France). Total RNA content and quality were evaluated by measuring optical density at 260 and 280 nm. Real-time RT-PCR was performed using iScript one-Step Real-time reverse transcription-polymerase chain reaction (qRT-PCR) Kit with SYBRgreen (Bio-Rad, Marnes-la-Coquette, France). The following primers were used for PCR: For LOXL1. Forward 5′-GACTTCGGCAACCTCAAGC-3′ Reverse 5′-TGTTGCAGAAACGTAGCGAC-3′ For elastin. Forward 5′-GTGTATACCCAGGTGGCGTG-3′ Reverse 5′-CGAACTTTGCTGCTGCTTTAG-3′ All primers were in separate zone of the exon. Amplifi cation was performed with 40 cycles, measuring the fl uorescence at the end of each cycle. The comparative Ct method (f¢Ct) was used for relative comparison. Real-time PCR experiments were calibrated with actin as the housekeeping gene. As negative controls, samples without RNA were used in the same conditions. Results were expressed related to control (untreated irradiated cells) and normalized to actin. Results and statistics. The results are expressed as percentage compared with untreated con- trol and then expressed as mean ± standard deviation (SD) from nine replicates. The statis- tical analysis was carried out using a Student t-test. IMMUNOHISTOCHEMISTRY: ELAFIN IN NORMAL HUMAN BIOPSIES EXPOSED TO UVA Cell culture. Human biopsies (abdominal part) from a 27-year-old donor were cultured in a specifi c defi ned medium at 37°C, with 5% CO2, for 10 d. The biopsies were either ir- radiated or not with UVA at 5 J/cm2 during a period of 10 d. UVA were applied from day 2 with and without our specifi c Hamamelis extract at 0.5%, every day in a topical or systemic manner. Assay method. Samples were fi xed in a formalin solution and they were then dehy- drated and embedded in paraffi n. Seven-micrometer sections were deparaffi nized.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)