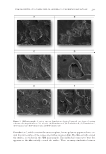
JOURNAL OF COSMETIC SCIENCE 210 Figures 2A and C and 3A and C due to the absence of the fi lm former which typically appears on the surface of the corneocytes and is not present in these formulations. This confi rms the fact that the sunscreen phase blends well with the corneocytes. Formulation D represents a typical sun care spray formulation, which contained a sun- screen phase and a relatively low level (1% w/w) of fi lm former. As expected, the sun- screen phase formed a continuous fi lm over the corneocytes. The fi lm former did not form the same type of network that we observed when the polymer was used alone (Figure 3B) but rather formed discrete blotches on the surface of the sunscreen fi lm. This behavior confi rms that there is an interaction between the sunscreen phase and the fi lm former, which indicates that portions of the fi lm former intercalate the sunscreen fi lm, whereas the remaining portion of the fi lm former creates a fi lm over the sunscreen layer. In Formulations E and F, two distinct polymers were added at low concentrations to study the interaction of composite fi lm formers on the surface characteristics of the fi lms created. Specifi cally, acrylates/dimethicone copolymer and hydroxypropyl cellulose, re- spectively, were added to Formulations E and F. By examination of the surface topography of the fi lms created, we observed that the surface properties of the two fi lms were com- pletely different. In the case of acrylates/dimethicone copolymer, there was no interaction of this polymer with the existing VA/butyl maleate/isobornyl acrylate copolymer. In fact, it appeared that acrylates/dimethicone copolymer formed discrete particles on the surface of the fi lm. On the other hand, the addition of hydroxypropyl cellulose to the existing polymer (VA/butyl maleate/isoburnyl acrylate copolymer) created a very defi ned network of the two polymers on the surface of the sunscreen fi lm. In an effort to study the applicability of this methodology to other formulations, we ex- amined the fi lm morphology of an SPF 30 commercial sunscreen containing VA/butyl maleate/isobornyl acrylate copolymer as a polymeric fi lm former. The micrographs of the commercial sunscreen and Formulation D are displayed in Figure 4. Examination of the two micrographs indicates a number of similarities between the two fi lms created. In both instances, the polymer formed a fi lm over the sunscreen fi lm, and the polymeric fi lm is made up of discrete particles rather than a network. The behavior of the fi lm former was Figure 4. SEM micrographs of a commercial sun care product containing VA/butyl maleate/isobornyl acrylate copolymer deposited (sprayed) onto layers of stratum corneum cells (magnifi cation = ×300).
FILM PROPERTIES OF POLYMERS USED IN ANHYDROUS SUNSCREEN FORMULATIONS 211 similar to the results already presented in this report, as well as additional unpublished data, and was independent of the composition of sunscreen phase. The effect of polymer concentration on the fi lm characteristics was also studied. We in- creased the concentration of polymer in Formulation D from 1%–3% (w/w). An SEM micrograph taken of this composition is displayed in Figure 5, which clearly demon- strates that the polymer formed a very defi ned fi lm over the corneocyte surface. The fi lm characteristics were quite similar, but because of the increased concentration of the fi lm former to 3% (w/w), there was better surface coverage. Figure 5 also displays a 3D micro- graph of the fi lm formed over the surface of the skin and sunscreen phase. In summary, this method confi rms earlier fi ndings that a polymeric fi lm typically forms over/above the sunscreen fi lm when an anhydrous sunscreen is sprayed on the skin (5). Because the polymeric fi lm is present as the uppermost layer on the skin, it will not only infl uence water resistance but will also affect the aesthetics of formulations. This makes selection of the correct polymer or polymer combination, as well as their levels in the formulation, of paramount importance. DISCUSSION The work presented in this article is the result of several years of investigation. The meth- odology presented appears quite simple and straightforward but the authors investigated many other methods and substrates that did not provide the same clarity and visuals. Among substrates investigated, we conducted studies with Vitro Skin™, silicone elasto- mers, and pig skin. None of these substrates had similar surface energy or topology like human corneocytes. In addition, standardization of the methodology was quite important to achieve reproducible results. All formulations (except the commercial control) were sprayed from the same size and type can/nozzle and contained the same propellant and were pressurized similarly. Spray rate and velocity were standardized as well. From an imaging stand point, multiple images were captured from each sample to ensure repro- ducibility of the methodology. The work presented in this article elucidated mechanistic information about polymer behavior in sunscreen formulation. One striking myth that this article uncovered is that Figure 5 . SEM micrographs of Formulation D with an adjusted concentration of 3% (w/w) VA/butyl maleate/isobornyl acrylate copolymer. (A) Conventional backscatter and (B) 3D images are shown at ×120 and ×250, respectively. The 3D image can be viewed with red and cyan 3D glasses.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)