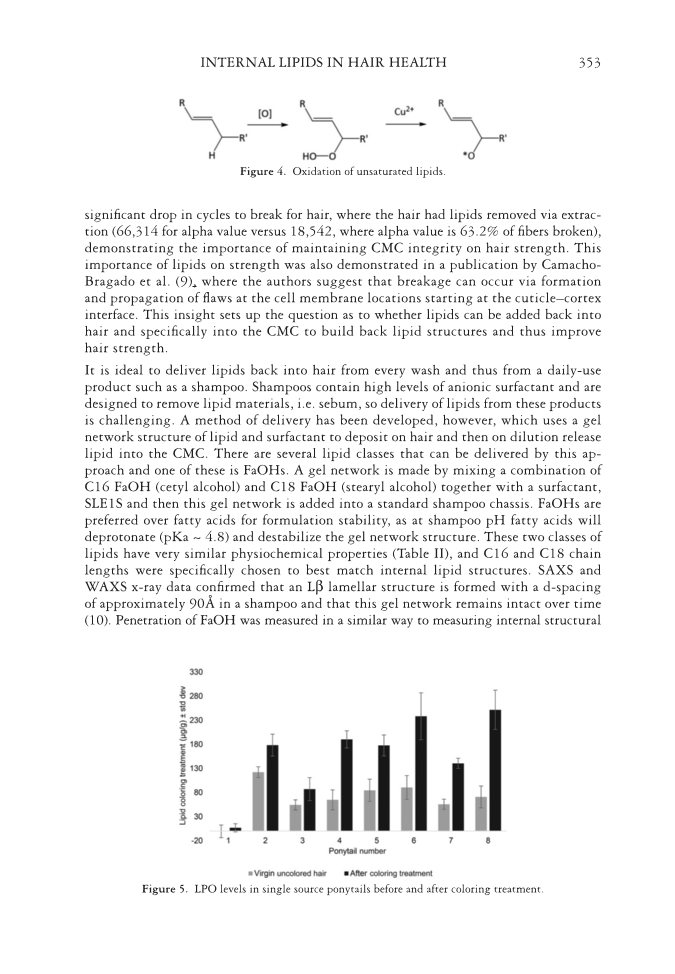
JOURNAL OF COSMETIC SCIENCE 352 and LPO levels after a 30 min colorant. LPOs were measured in almost all the starting ponytails but levels varied between ponytails. It is predicted that UV light can also form LPOs (see additional evidence below), so it is reasonable to assume these LPOs have formed from previous UV exposure by the women who donated the ponytails and may explain why levels vary from person to person. There is an increase in LPOs for all the ponytails tested apart from #1 (which had very low starting levels), providing evidence that this is a plausible mechanism for loss of unsaturated lipids after coloring. This loss is demonstrated in Figure 6, where three additional ponytails were assessed for unsatu- rated fatty acid levels after a 30-min coloring treatment. All three ponytails show a signifi cant loss of unsaturated fatty acids after treatment. The same eight ponytails were also exposed to UV irradiation that mimicked sunlight for 20 h Figure 7 shows change on LPO levels for each of the ponytails with some LPO levels increasing and some decreasing. In this case, these data illustrate that LPOs can not only be formed via UV oxidation but also be destroyed by UV oxidation. The perhydroxyl bond O-OH can be broken directly via UV absorption or more likely it reacts with other ROS formed during UV exposure. One pathway for subsequent reactivity of LPOs is shown in Figure 4 where redox metals, such as copper, can react with organic peroxides to form highly reactive alkoxyl radicals, which will react further with lipids and protein structures. Previously published work has shown such mechanisms occur with hair dosed with high versus low copper levels, with added copper and UV exposure measured LPO levels are lower than with low copper levels (7). The importance of internal structural lipids for the structural integrity of hair has been demonstrated by McMullen et al. (8), who showed removing lipids via solvent extraction caused differences in moisture management, interaction with cationic polymers and sur- factants, and impact on style hold. Fatigue measurements using fi xed weights showed a Table I L oss of lipids with washing (100 cycles) Hair treatment Sat. fatty acids (μg/g) Unsat. fatty acids (μg/g) Wax esters (μg/g) Ceramides (μg/g) Chol. + Chol. sulfate (μg/g) Total (μg/g) Vigin 8,391 ± 779 4,522 ± 252 932 ± 50 283 ± 8 1,187 ± 150 15,308 ± 1,134 +100 Wash 7,204 ± 429 2,903 ± 140 323 ± 14 293 ± 16 994 ± 70 11,727 ± 548 5 × Colored 7,312 ± 234 2,815 ± 151 404 ± 56 277 ± 19 1,108 ± 84 11,934 ± 340 +100 Wash 6,382 ± 884 2,215 ± 168 269 ± 13 269 ± 10 1,027 ± 38 10,195 ± 977 Figu re 3. Lipid loss from root to tip.
INTERNAL LIPIDS IN HAIR HEALTH 353 Figur e 4. Oxidation of unsaturated lipids. Figure 5. LPO levels in single source ponytails before and after coloring treatment. signifi cant drop in cycles to break for hair, where the hair had lipids removed via extrac- tion (66,314 for alpha value versus 18,542, where alpha value is 63.2% of fi bers broken), demonstrating the importance of maintaining CMC integrity on hair strength. This importance of lipids on strength was also demonstrated in a publication by Camacho- Bragado et al. (9), where the authors suggest that breakage can occur via formation and propagation of fl aws at the cell membrane locations starting at the cuticle–cortex interface. This insight sets up the question as to whether lipids can be added back into hair and specifi cally into the CMC to build back lipid structures and thus improve hair strength. It is ideal to deliver lipids back into hair from every wash and thus from a daily-use product such as a shampoo. Shampoos contain high levels of anionic surfactant and are designed to remove lipid materials, i.e. sebum, so delivery of lipids from these products is challenging. A method of delivery has been developed, however, which uses a gel network structure of lipid and surfactant to deposit on hair and then on dilution release lipid into the CMC. There are several lipid classes that can be delivered by this ap- proach and one of these is FaOHs. A gel network is made by mixing a combination of C16 FaOH (cetyl alcohol) and C18 FaOH (stearyl alcohol) together with a surfactant, SLE1S and then this gel network is added into a standard shampoo chassis. FaOHs are preferred over fatty acids for formulation stability, as at shampoo pH fatty acids will deprotonate (pKa ~ 4.8) and destabilize the gel network structure. These two classes of lipids have very similar physiochemical properties (Table II), and C16 and C18 chain lengths were specifi cally chosen to best match internal lipid structures. SAXS and WAXS x-ray data confi rmed that an Lβ lamellar structure is formed with a d-spacing of approximately 90Å in a shampoo and that this gel network remains intact over time (10). Penetration of FaOH was measured in a similar way to measuring internal structural
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)