JOURNAL OF COSMETIC SCIENCE 366 DESORPTION OF WATER ON HAIR Triplicates of 2 g of virgin and bleached hair were placed in Eppendorf tubes (1.5 mL) and left to hydrate in a desiccator fl ask with distilled water in the bottom (RU 98–99% at 21°C) for 20 d. The hydrated hair mass was obtained in an analytical scale and placed in a desiccator with P2O5 (RU 0% at 21°C) (7) and the mass variation was acquired over the time. RESULTS AND DISCUSSION DSC ANALYSIS The DSC peak is associated with the temperature of denaturation of the hair keratinous materials. The area under the curve of the peak corresponds to the enthalpy of degrada- tion of the helical structures, represented by delta H (8). It is correlated with quantity and quality of the α-helical materials of the intermediate fi laments (IFs) of the hair (8–10). The obtained values for enthalpy variation and peak temperature from DSC analysis are presented in Table II. The numbers suffered important reductions because of the bleach- ing process in both parameters. Both variations have been associated by Wortmann et al. (8) to the loss of helical fi brous IFs and intermediate fi lament-associated proteins (IFAPs) caused by the bleaching process being the fi rst one even more affected, which explains the higher discrepancy of fi gures in the comparison of the virgin and the bleached hair. FLUORESCENCE MICROSCOPY ANALYSIS The level of damage of the hair can infl uence the fl uorescence intensity detected as rhoda- mine B presents higher affi nity to negative sites of the damaged hair. The bleached hair, therefore, is expected to present higher intensities than the virgin hair as the presence of sulfonic acid generated from the break of the disulfi de bonds is much more expressive (3). The fl uorescence intensity was calculated by the Image Analysis software in each captured image and then compared between the types of hair. In the fl uorescence microscopy analysis, the virgin hair presented values of fl uorescence intensity lower than three luminance as bleached hair presented fi gures fi ve times higher (Table II). These results are in accordance with the ones found by Pötsch and Moeller (11). Low fl uorescence intensity was evidenced in the virgin hair in investigations with rhoda- mine B as well, whereas damaged hair presented higher degrees of intensity. Table II Results Obtained for DSC Analysis and Fluorescence Microscopy for the Virgin and the Bleached Hair Type of hair H (J/g) Peak T (°C) Fluorescence intensity (Lum) Virgin 12.4777 ± 0.3579 147.6347 ± 0.5764 1,6415 ± 1,2177 Bleached 9.4066 ± 0.3407 141.9717 ± 0.5691 16,3907 ± 1,1158 Mean ± confi dence interval.
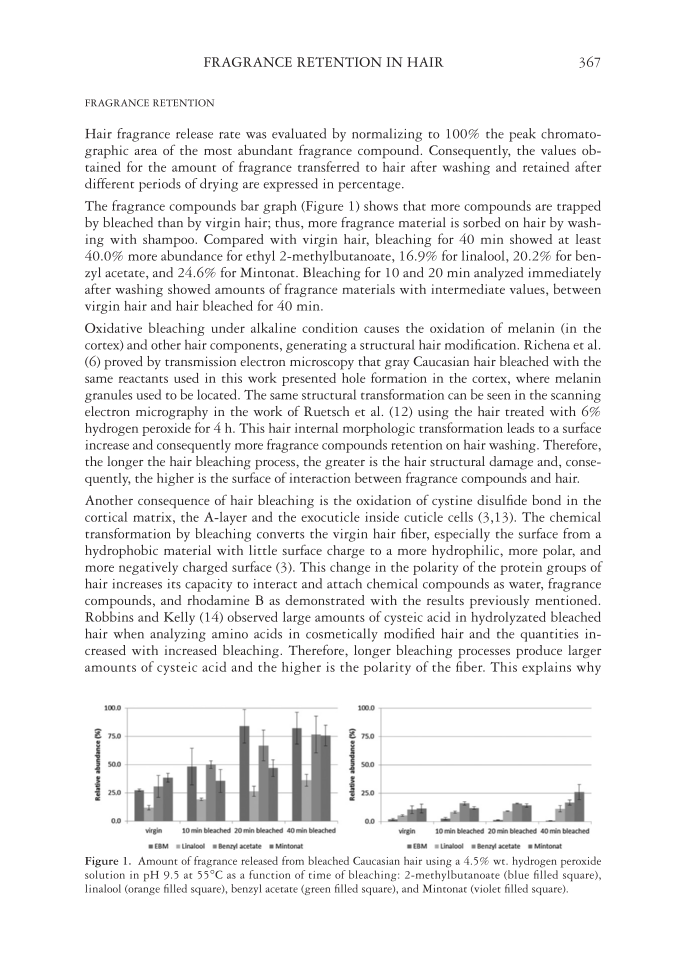
FRAGRANCE RETENTION IN HAIR 367 FRAGRANCE RETENTION Hair fragrance release rate was evaluated by normalizing to 100% the peak chromato- graphic area of the most abundant fragrance compound. Consequently, the values ob- tained for the amount of fragrance transferred to hair after washing and retained after different periods of drying are expressed in percentage. The fragrance compounds bar graph (Figure 1) shows that more compounds are trapped by bleached than by virgin hair thus, more fragrance material is sorbed on hair by wash- ing with shampoo. Compared with virgin hair, bleaching for 40 min showed at least 40.0% more abundance for ethyl 2-methylbutanoate, 16.9% for linalool, 20.2% for ben- zyl acetate, and 24.6% for Mintonat. Bleaching for 10 and 20 min analyzed immediately after washing showed amounts of fragrance materials with intermediate values, between virgin hair and hair bleached for 40 min. Oxidative bleaching under alkaline condition causes the oxidation of melanin (in the cortex) and other hair components, generating a structural hair modifi cation. Richena et al. (6) proved by transmission electron microscopy that gray Caucasian hair bleached with the same reactants used in this work presented hole formation in the cortex, where melanin granules used to be located. The same structural transformation can be seen in the scanning electron micrography in the work of Ruetsch et al. (12) using the hair treated with 6% hydrogen peroxide for 4 h. This hair internal morphologic transformation leads to a surface increase and consequently more fragrance compounds retention on hair washing. Therefore, the longer the hair bleaching process, the greater is the hair structural damage and, conse- quently, the higher is the surface of interaction between fragrance compounds and hair. Another consequence of hair bleaching is the oxidation of cystine disulfi de bond in the cortical matrix, the A-layer and the exocuticle inside cuticle cells (3,13). The chemical transformation by bleaching converts the virgin hair fi ber, especially the surface from a hydrophobic material with little surface charge to a more hydrophilic, more polar, and more negatively charged surface (3). This change in the polarity of the protein groups of hair increases its capacity to interact and attach chemical compounds as water, fragrance compounds, and rhodamine B as demonstrated with the results previously mentioned. Robbins and Kelly (14) observed large amounts of cysteic acid in hydrolyzated bleached hair when analyzing amino acids in cosmetically modifi ed hair and the quantities in- creased with increased bleaching. Therefore, longer bleaching processes produce larger amounts of cysteic acid and the higher is the polarity of the fi ber. This explains why Figure 1. Amount of fragrance released from bleached Caucasian hair using a 4.5% wt. hydrogen peroxide solution in pH 9.5 at 55°C as a function of time of bleaching: 2-methylbutanoate (blue fi lled square), linalool (orange fi lled square), benzyl acetate (green fi lled square), and Mintonat (violet fi lled square).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)