DETERMINATION OF SUNSCREEN AGENTS BY HPLC AND CHEMOMETRIC ANALYSIS 169 In anot her recent study, Wharton et al. (19) used a 3-μm Hypersil BDS C18 column for the simultaneous determination of seven UV fi lters. The mobile phase was used in a gra- dient method consisting of ethanol and 1% acetic acid. In gener al, most of the reported methods dealing with the determination of a large num- ber of sunscreen compounds by HPLC recommend the use of binary, ternary, or quater- nary solvent mixtures as the mobile phase. Gradient elutions have been used to obtain adequate resolution in the separation of a large number of UV fi lters as they allow a wide range of solvent polarity. However, this type of elution has the disadvantage of requiring longer analysis time and higher costs. Although the high boiling points of UV fi lters made them less suited for gas chromatogra- phy (GC) analysis, several successful examples of identifi cation and quantitation (20-24) by this technique have been described. A method was developed by Haunschmidt et al. (25) based on direct analysis in real-time mass spectrometry for the qualitative and semiquantitative analysis of eight organic UV fi lters and four parabens in 12 cosmetic products with substantially different formula- tions (such as cream, milk, lotion, oil, and lipstick). The aim of this study was to develop a fast, simple, and practical HPLC method using phosphoric acid with distilled water and ethanol to determine the most widely used UV fi lters in cosmetics commercialized in Iran. The method was validated and applied for the determination of nine sunscreens in formulations (lotions) commercially available in Iran to verify if they are in conformity with the current legislation. An established extraction technique using ethanol was applied to eliminate the possible interaction effects between various ingredients used in sunscreens. The stability of UV fi lters was assessed before method development. All the agents were kept away from light and heat. METHOD A ND MATERIAL INSTRUME NTATION A Knauer ® K-1000 liquid chromatograph, which was equipped with a mixing chamber, 20-μl loop, degasser, and Knauer® K-2500 UV detector (Knauer, Berlin, Germany), was applied. To separate substances, an Agilent C18 (4.6 × 150 mm, 5 μm) column was used. Table I Gradient Timetable Used for the Mobile Phase Time (min) Flow (ml/min) Solvent A (%) Solvent B (%) 0 1 30 70 18 1 25 75 22 1 0 100 30 1 0 100 35 1 30 70 Temperature of the column was set at 40°C. The injected volume was 20 μl. The wavelength was set at 312 nm. Because of the overlap of the BMDBM chromatogram with the EHMC chromatogram, another wavelength (390 nm) on which only BMDBM had absorbance was chosen. To compute the exact amount of EHMC, the area seen at 390 nm should be subtracted from the area seen at 312 nm (which is related to both substances).
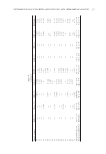
JOURNAL OF COSMETIC SCIENCE 170 STANDARDS AND R EAGENTS The chemical st andards include BMDBM, 3-benzophenone (3-Benz), MBC, octocrylene (OCT), PABA, EHMC, HMS, EHS, and EHT. All were purchased from Sigma-Aldrich (St. Louis, MO). The solvents were prepared by Merck (HPLC-grade high-purity product of Merck, Darmstadt, Germany). Figure 1. Separation o f nine sunscreen agents at 312 nm (3-Benz: 3-benzophenone, OCT: octocrylen).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)