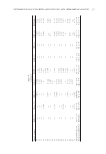
DIRECT ANALYSIS OF DIMETHICONE IN AQUEOUS EMULSIONS 215 concentrations while still in aqueous emulsions. This rapid method of analysis will be helpful in quality testing to support manufacturing and stability testing of products that contain dimethicone emulsions. Direct analysis of samples without sample preparation is appealing compared with more lengthy, complex methods. Of course, as with all new analytical procedures, this method should be validated on a product-by-product basis because other ingredients and excipients could potentially interfere with this procedure. ACKNOWLEDGMENT This work was supported b y the Center for Chemical Technology at Weber State University. REFERENCES (1) “Dimethic one,” Toxic o logy Data Network, U.S. National Library of Medicine, accessed April 8, 2019, https://toxnet.nlm.nih.gov /cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+1808. (2) P. K. Farris, “Topica l Skin Care and the Cosmetic Patient,” in Master Techniques in Facial Rejuvenation, (Elsevier Health Sciences, Atlanta, GA, 2018), pp. 68–72. (3) E. S. Ihde, J. R. B os camp, J. M. Loh, and L. Rosen, Safety and effi cacy of a 100% dimethicone pedicu- locide in school-age children, BMC Pediatr., 15, 70–76 (2015). (4) J. Heukelbach, D. P il ger, F. A. Oliveira, A. Khakban, L. Ariza, and H. Feldmeier, A highly effi cacious pediculicide based on dimethicone: randomized observer blinded comparative trial, BMC Infect Dis., 8, 115–124 (2008). Table IV Effect of Various Excipients on Method 2 Assay Results Excipient added Excipient conc. (%) Dimethicone % averagea (n = 3) RSD (%) Control 0.00 3.01 0.51 Laureth-4 0.30 2.96 1.60 0.60 3.02 2.32 Laureth-23 0.30 3.05 1.68 0.60 3.06 1.68 Triton X-100 0.30 2.95 1.55 0.60 3.03 2.70 PPG-15 stearyl ether 0.30 3.00 2.03 0.60 3.01 2.03 PEG-100 stearate 0.30 3.01 2.82 0.60 2.95 1.74 Sorbitan laurate 0.30 3.02 2.82 0.60 2.95 1.60 Methylparaben 0.05 2.99 1.65 0.10 2.99 0.84 Propylparaben 0.05 3.01 0.96 0.10 3.00 1.17 Phenoxyethanol 0.05 2.99 0.67 0.10 3.00 1.07 DMDM hydantoin 0.01 3.00 1.68 0.02 3.02 1.15 Kathon 0.0050 2.97 0.19 0.0100 3.00 1.39 Sodium benzoate 0.05 3.01 1.45 0.10 3.00 1.15 a Actual dimethicone concentration in all samples = 3.00% (g/100 g) .
JOURNAL OF COSMETIC SCIENCE 216 (5) Skin Protectant Dru g Products for Over-The-Counter Human Use Skin Protection, 21 C.F.R. § 347.10 (2018). (6) C. Flaviana da Silva Acunha and J. H. Zimnoch dos Santos, An analytical method for quantifying di- methicone in a 30% simethicone emulsion using gas chromatography, Br. J. Anal. Chem., 6, 278–285 (2011). (7) S. Andersson, D. A. Y oung, and S. Jacobssen, Quantitation of polydimethylsiloxane in pharmaceutical formulations by gel permeation chromatography, J. Chromatogr. A, 477, 474–476 (1989). (8) D. E. Moore, T. X. Li u, W. G. Miao, A. Edwards, and R. Elliss, A RP-LC method with evaporative light scattering detection for the assay of simethicone in pharmaceutical formulations, J. Pharm. Biomed. Anal., 30, 273–278 (2002). (9) L. Sandford and M. Wo odman, “UHPLC Analysis of Dimethicone (Polydimethylsiloxane) by Gradient Elution with ELSD,” HPLC2016, (Agilent Technologies Inc., San Francisco, CA, 2016), accessed April 8, 2019, https://www.agilent.com/cs/library/posters/public/HPLC2016_P-Th-2301_Dimethicone.pdf. (10) Dimethicone Applica t ion Note #0048E, (Alltech Associates, Inc., Deerfi eld, IL, 2000), accessed April 8, 2019, https://docslide.net/documents/note-0048e-dimethicone-associates-inc-2051-waukegan-road- deerfi eld.html. (11) J. J. Jadhav, S. Mu n gekar, J. V. Velada, H. A. Doshi, V. Gajbe, and K. Raunak, A simple and rapid HPLC method for estimation of Dimethicone from formulations, Indian Drugs, 50, 26–29 (2013). (12) J. V. Gruber, B. R . Lamoureux, N. Joshi, and L. Moral, The use of x-ray fl uorescent spectroscopy to study the infl uence of cationic polymers on silicone oil deposition from shampoo, J. Cosmet. Sci., 52, 131–136 (2001). (13) H. M. Haake, H. La gr en, A. Brands, W. Eisfeld, and D. Melchior, Determination of the substantivity of emollients to human hair, J. Cosmet. Sci., 58, 443–450 (2007). (14) E. G. Gooch, Deter mi nation of traces of silicone defoamer in fruit juices by solvent extraction/atomic absorption spectroscopy, J. AOAC Int., 76, 581–583 (1993). (15) M. Sabo, J. Gross, a nd I. E. Rosenberg, Quantitation of dimethicone in lotions using Fourier transform infrared spectral subtraction, J. Soc. Cosmet. Chem. 35, 273–281 (1984). (16) A. Rohman, A. Musfi ro h, and E. G. Wijaya, Quantitative determination of simethicone in antacid sus- pension and chewable tablet using FTIR Spectroscopy, Global J. Pharmacol., 7, 270–275 (2013). (17) G. Torrado, A. Garcı a- Arieta, F. de los Rıos, J. C. Menendez, and S. Torrado, Quantitative determina- tion of dimethicone in commercial tablets and capsules by Fourier transform infrared spectroscopy and antifoaming activity test, J. Pharm. Biomed. Anal., 19, 285–292 (1999). (18) E. B. Walker, D. R. Dav ie s, and M. Campbell, Quantitative measurement of trans-fats by infrared spec- troscopy, J. Chem. Ed., 84, 1162–1164 (2007). (19) M. E. Miller, L. P. McK in non, and E. B. Walker, Quantitative measurement of metal chelation by Fou- rier transform infrared spectroscopy, Anal. Chem. Res., 6, 32–35 (2015). (20) Dimethicone Monograph, Un ited States Pharmacopeia/National Formulary, USP24/NF19, 2448– 2449 (2000). (21) “Silicones in Industria l Applications,” in Inorganic Polymers, R. De Jaeger and M. Gleria. Eds. (Nova Science Publishers, Hauppauge, NY, 2007), pp. 61–163. (22) V. Jankauskaitė, P. Narmo n tas, and A. Lazauskas, Control of polydimethylsiloxane surface hydrophobic- ity by plasma polymerized hexamethyldisilazane deposition, Coatings, 9, 36–43 (2019).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)