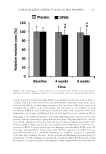
JOURNAL OF COSMETIC SCIENCE 292 persisting infl ammation (4,5). Even the most gentle methods may leave some pigment traces in the form of shades or blurred spots. These problems may push patients to add new tattoos over leftovers of the old ones to hide these cosmetic defects. T herefore, it is obvious that the need of complete removal of old tattoo pigments with minimal skin damage is undoubtedly acute. This need will become even greater when tattoo carriers decide that their tattoo pictures should be removed or corrected. In this context, we consider possible approaches that may lead to new solutions to be used in the technologies of tattoo removal. O rganic tattoo pigments are particles usually sized from 200 nm to 5 μm (6), which are electrophoretically mobile. Their electrokinetic (zeta) potential can be determined using appropriate methods such as the classical measuring of the difference in liquid levels reached in the U-shaped pipe after application of some fi xed external DC-voltage during some period of time. This method is still extensively used in many fi elds of applied tech- nique, chemistry, and biology (7–9). Th en, when the determined zeta potential value is suffi cient to expect any substantial mobility of the particles, the possibility of tattoo removal can be tested further in direct experiments involving a model of the human skin and, fi nally, be verifi ed in the experi- ments with real skin in volunteers. Th erefore, it is important to determine some typical zeta potentials of the tattoo pigments in isotonic solutions. MA TERIALS AND METHODS PI G MENTS A series of unbranded dyes for tattooing (Figure 1) was purchased at an online store and then used for the preparation of the solutions and other mixtures to investigate their electrophoretic mobility. Only four color samples: black, white, red, and green were selected for this investigation because they are the most popular for tattoo pictures. PR EPARATION OF PIGMENT SUSPENSIONS Al l the pigments were suspended in isotonic solution (0.9% NaCl) to reach the concen- trations described in the corresponding manuals (0.25 mL/L). As a result, four brightly colored solutions were obtained and then each of them was placed in the U-shaped glass tube (Figure 2, 1) for measuring its zeta potential. DE TERMINATION OF THE ζ POTENTIAL Fol lowing the classical method of determination (10), each solution was poured into the tube in such a way to fi ll it over the main faucets (Figure 2, 2) level. Then both faucets were closed and excessive colored solution removed from the upper parts of the device (Figure 2, 3), which then was fi lled with the coupling fl uid (same isotonic solution of
ELECTROPHORETIC MOBILITY OF SOME TATTOO DYES 293 NaCl containing no dissolved dyes). Finally, the balancing faucet (Figure 2, 4) was opened to equalize the coupling fl uid levels in both parts of the tube. Aft erward, both extremities of the U-shaped tube were connected electrically to the power supply through additional agar–agar bridges installed between each bend of the tube and the power supply outputs. Then the main faucets (2) were opened and voltage was applied between both parts of the glass tube. Bec ause the dye grains acquire some electric charge in the isotonic solution, they will move toward one of the poles causing a level difference between working and coupling liq- uids. Having measured the difference between the margins in each bend, the zeta poten- tial can be calculated by the following formula (11): ζ = ηhl Eεε 0 t, w her e η is the viscosity of working solution (because all working solutions were quite diluted, it was taken as equal to the viscosity of water 1 × 10-3 Pa s) h is the fi nal differ- ence between the solution levels in each bend, m l is the distance between the axes of two parts of the U-shaped tube (0.58 m) E is the electric voltage applied (260 V throughout all experiments) ε 0 is the standard vacuum dielectric permittivity constant (8.85 × 10-12 F/m) ε is the relative dielectric permissivity of water (equal to 81) and t is the duration of electrophoresis (s). Figure 1. Samples of tattooing pigments used for investigation of the electrophoretic activity.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)