JOURNAL OF COSMETIC SCIENCE 310 Palmitic a cid molecules form dimers because of OH….O hydrogen bonds and these di- mers pack into bilayers (22,23). Palmitic acid has a small polar head group (–COOH). Changing the head group to bulky KTTKS probably imposes some degree of disorder into the structure. Besides this, the molecules become longer with higher d-spacing: 34.6 Å for palmitic acid versus 44.2 Å for Pal-KTTKS. Pal-KTTKS also shows a higher d-spacing than KTTKS that are defi nitely due to the presence of long chain (C16) in the structure. Based on the results of partitioning and solubility tests, KTTKS has a highly hydrophilic nature (logP 0 and high water solubility). Because higher lipophilicity is required for good permeation through the skin (logP 0–3), KTTKS, like many other peptides such as Ala–Ala–Pro–Val (24), tetragastrin (25), and TRH (26), is not a good candidate for skin delivery. The results of the in vitro skin permeation study of KTTKS, which showed KTTKS did not permeate across the skin (27), is in good agreement with the present fi nding. KTTKS is a p entapeptide without a hydrophobic tail, but Pal-KTTKS has a 16-carbon chain (palmitic acid) as the hydrophobic tail, so Pal-KTTKS is a peptide amphiphile. The peptide amphiphiles are capable of forming a diversity of aggregates, such as micelles, cylindrical fibrils, sheets, and vesicles (28). Here, the results of tensiometry indicate that Pal-KTTKS has surface activity and reduces the surface tension of water to 50.3 ± 0.4 mN/m. The CMC of Pal-KTTKS in water was also determined by the ring method and found to be 0.024 ± 0.004 mM. There are no reports on the CMC value of Pal-KTTKS in aqueous solutions using the ring method. This CMC value resembles polysorbate 80 (0.02–0.03 mM) (29), which is structurally similar to Pal-KTTKS. The CMC value could be considered as the solubility of the individual molecules with surface activity because above this concen- tration, the molecules are not dissolved in the monomeric forms and aggregate as micelles (30). As said, this value for Pal-KTTKS was 0.024 ± 0.004 mM or 19.25 ± 2.9 mg/L thus, considering only individual molecules, it could be said this peptide amphiphile is practically insoluble in water. Cycled DSC and TG A results show that both peptide powders are hygroscopic. The hy- groscopic nature of some peptides, especially when present as porous lyophilized pow- ders, is a well-known property. On the other hand, it has been argued that peptides containing charged amino acids (such as arginine, aspartic acid, glutamic acid, histidine, and lysine) are hygroscopic (31). KTTKS and its derivative have two lysine amino acids, and therefore, it is possible that they absorb water. Hence, two water-related transitions (T1 KTTKS and T1 Pal-KTTKS ) in their DSC thermograms disappear on reheating, which is in agreement with weight losses observed in TGA thermograms. Finally, as far a s formulation is concerned, techniques such as UV, XRD, polarized light microscopy, SEM, and thermal analysis can be used to monitor changes during pharmaceu- tical processing, stability studies, and storage by the formulator. Some of these techniques, e.g., DSC, can be used to determine drug–excipients interactions. During pharmaceutical processing and storage, a formulator should be aware of the polymorphism and the pos- sibility of change of a polymorphic form into another due to processing (such as mixing and heating) and storage. For example, if a formulator uses these substances in the sus- pension dosage form (the presence of solid particles in the formulation), the stability during storage can be monitored using XRD or polarized light microscopy, as described earlier. The same applications apply to the SEM morphologies. SEM and polarized light
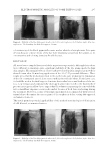
PREFORMULATION STUDIES OF PEPTIDES KTTKS AND PAL-KTTKS 311 represent crystal habits as well. In this regard, some habits such as needle-likes might provide some degrees of discomfort in topical applications and should be considered in the formulation and stability studies by the formulator. CONCLUSION Here, KTTKS and P al-KTTKS were synthesized and the physicochemical properties of both peptides and, therefore, the effects of covalent attachment of palmitic acid on KTTKS properties were investigated. In short, these peptides showed birefringence, irregular morphology, wide particle size distribution, and no melting point before decomposition. In addition, KTTKS was very hydrophilic in nature, and its aqueous solution remains stable at 32°C for over 48 h. In terms of internal structure, Pal-KTTKS appeared more ordered than KTTKS. Micelle formation of Pal-KTTKS in water occurs at low concentra- tions. The results show that fatty acid attachment to KTTKS causes some changes in chemical properties (such as solubility and partition coeffi cient), whereas physical properties (such as morphology, thermal behavior, and birefringence) are not affected so much. These results can be used for formulation of these peptides for topical delivery or any other drug delivery system development. The effects of palmitic acid conjugation on KTTKS prop- erties might be used for the preparation of palmitic acid derivatives on other peptides. ACKNOWLEDGMENTS This paper is a part of PhD thesis of Seyedeh Maryam Mortazavi at the School of Pharmacy, Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran and was fi nancially supported by SBMU. REFERENCES (1) M. Ramos-e-Si l va, L. Celem, S. Ramos-e-Silva, and A. P. Fucci-da-Costa, Anti-aging cosmetics: facts and controversies, Clin. Dermatol., 31, 750–758 (2013). (2) N. H. Abu Sam a h and C. M. Heard, Topically applied KTTKS: a review. Int. J. Cosmet. Sci., 33, 483– 490 (2011). (3) S. Schagen, T o pical peptide treatments with effective anti-aging results, Cosmetics, 4, 16 (2017). (4) F. Gorouhi an d H. I. Maibach, Role of topical peptides in preventing or treating aged skin, Int. J. Cosmet. Sci., 31, 327–345 (2009). (5) K. Katayama, J . Armendariz-Borunda, R. Raghow, A. H. Kang, and J. M. Seyer, A pentapeptide from type I procollagen promotes extracellular matrix production, J. Biol. Chem., 268, 9941–9944 (1993). (6) C. Lu, B. M. K im, D. Lee, M. H. Lee, J. H. Kim, H. B. Pyo, and K. Y. Chai, Synthesis of lipoic acid- peptide conjugates and their effect on collagen and melanogenesis, Eur. J. Med. Chem., 69, 449–454 (2013). (7) L. R. Robinso n , N. C. Fitzgerald, D. G. Doughty, N. C. Dawes, C. A. Berge, and D. L. Bissett, Topical palmitoyl pentapeptide provides improvement in photoaged human facial skin, Int. J. Cosmet. Sci., 27, 155–160 (2005). (8) J. J. Fu, G. G . Hillebrand, P. Raleigh, J. Li, M. J. Marmor, V. Bertucci, P. E. Grimes, S. H. Mandy, M. I. Perez, S. H. Weinkle, and J. R. Kaczvinsky, A randomized, controlled comparative study of the wrinkle reduction benefi ts of a cosmetic niacinamide/peptide/retinyl propionate product regimen vs. a prescription 0.02% tretinoin product regimen, Br. J. Dermatol., 162, 647–654 (2010). (9) G. B. Fields a nd R. L. Noble, Solid phase peptide synthesis utilizing 9-fl uorenylmethoxycarbonyl amino acids, Int. J. Pept. Protein Res., 35, 161–214 (1990).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)