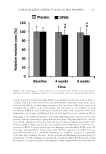
JOURNAL OF COSMETIC SCIENCE 308 decomposition. The temperature of maximum weight loss (230°C) obtained from the first derivative TGA thermograms is almost similar for both peptides (see Table III). DSC thermograms of KTTKS and Pal-KTTKS are illustrated in Figure 6A. As seen, both peptides exhibit endothermic transitions with a peak of 59.8 ± 1.3°C (T1 KTTKS ) for KTTKS and 50.6 ± 4.4°C (T1 Pal-KTTKS ) for Pal-KTTKS. Given the results obtained from the melting point studies, which showed that peptides did not show any sign of melting, these endothermic transitions could not be attributed to the melting of peptides and were assumed here to be water-related intrinsic structural rearrangement, polymorphism, or evaporation, as discussed later. KTTKS has another endothermic peak with low enthalpy (T2 KTTKS ) (see Figure 6A). A third endothermic transition (T3 KTTKS ) starts at 152.1 ± 3.9°C in the thermogram of KTTKS. This endotherm is in good agreement with the result of decomposition obtained from melting point studies (154°C). For Pal-KTTKS, the second endothermic transition (T2 Pal-KTTKS ), starting at 142.7 ± 0.9°C, could also be attributed to the fi rst decomposition of the peptide conjugate as observed in melting point studies (150°C). Visual observations of the DSC pan after removal revealed sever changes in the peptide appearance (change in color and texture). These changes might be considered as signs of peptide decomposition. To ascertain the nature of T1 KTTKS and T1 Pal-KTTKS , cycled DSC was performed. Cycled DSC experiment indicated that T1 KTTKS and T1 Pal-KTTKS disappeared in the second heat- ing run, whereas T2 KTTKS , T3 KTTKS , and T2 Pal-KTTKS remained unchanged (see Figure 6B). These results might show that T1 KTTKS and T1 Pal-KTTKS are related to water evapora- tion from peptides, as discussed later. To ensure the relationship of T1 KTTKS and T1 Pal-KTTKS to water evaporation, a cycled TGA experiment was performed here. As shown in Figure 5C and D, TGA weight losses for the fi rst heating runs are 5.9% and 3.3% for KTTKS and Pal-KTTKS, respectively. Table III Percent Weight Loss of Peptides and the Temperature of Maximum Weight Loss during TGA Study in the Range of 25–595°C Peptide name Weight loss (%) over three temperature ranges Temperature of maximum weight loss (°C) 25–150°C 150–250°C 250–595°C KTTKS 7.47 ± 0.84 45.34 ± 1.29 41.97 ± 1.03 233.92 ± 1.49 Pal-KTTKS 6.55 ± 0.99 33.61 ± 0.67 56.38 ± 0.67 229.26 ± 0.86 Data are represented as mean ± SD (n = 3). Figure 6. (A) Sample DSC thermograms o f KTTKS and Pal-KTTKS at a heating rate of 10°C/min over 25–165°C and (B) DSC thermograms of preheated–cooled KTTKS and Pal-KTTKS samples at a heating rate of 10°C/min over 25–165°C (see text for more explanation).
PREFORMULATION STUDIES OF PEPTIDES KTTKS AND PAL-KTTKS 309 In the second heating runs, weight losses were less than 1% for both peptides. After both heating processes in TGA, the pans containing peptides were inspected visually there were no macroscopic changes in the appearance of peptide powders. In agreement with the present results, some researchers (16) showed that a DSC transition of around 63.1°C in a lyophilized synthetic trapezoid-inspired peptide fragment disappeared in the second DSC heating run. It was concluded by investigators to be due to the presence of bound water and subsequent water loss in the second run. S TABILITY STUDIES Ensuring the stability of permeants during the skin permeation experiment is crucial. The concentration of KTTKS solution was 100 μg/mL at the beginning of the stability study. After 48 h, the concentration of KTTKS solution reached 99.3 ± 1.7 μg/mL. Thus, it could be concluded that KTTKS remains stable at 32°C at least for 48 h. Therefore, there is no concern about the instability of this permeant during the skin permeation studies for at least 48 h. DISC USSION KTTK S and its derivative are popular in the cosmetic industry because of their anti- wrinkle properties, but many of their physicochemical properties remain unknown. This study is an attempt to defi ne the physicochemical properties of these peptides and to in- vestigate the effects of covalent attachment of a fatty acid on peptide properties. In t he case of UV absorbance, λmax of both peptides was at less than 200 nm. KTTKS and Pal-KTTKS have no aromatic amino acids in their structure, and the UV absorbance of them is due to the peptide bonds. The photons are absorbed by peptide bond at the maximum wavelength of below 210 nm (17). Note that the wavelengths below 200 nm belong to vacuum UV (18). The UV radiation is forcefully absorbed by atmospheric oxygen in the vacuum UV range (19). Therefore, a free oxygen environment is required to accu- rately assay KTTKS and Pal-KTTKS if UV spectroscopy at the maximum wavelength is to be used. Because KTTKS and Pal-KTTKS possess a broad absorption peak, it might be said that it is possible to measure the absorption at the longer wavelength, e.g., at 205 nm, but we noted that at this wavelength, the UV radiation is absorbed by many compounds used in buffers (20), which are mainly applied in skin permeation studies. In X- ray studies, both peptides showed a broad peak (hump) at around 2θ = 19° (see Figure 4), which was absent in palmitic acid. Such a broad peak at a wide angle region, which has also been reported for other peptides (21), might indicate that these peptides contain either some amorphous structures or some disordered crystalline structures. Both peptides also show peaks at 2θ 10 that indicate some long-range orders. The presence of some degrees of orders in the structure was also confi rmed by showing birefringence in the cross-polarized light. The presence of new peaks in the XRD pattern of Pal-KTTKS, which is not present in the pattern of KTTKS, means that the structures of these peptides are not the same therefore, some possible changes in the physicochemical properties of peptides, such as solubility, dissolution rate, and fl owability, are expected and should be considered by formulators.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)