THE JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS This edition is published by THE SOCIETY OF COSMETIC CHEMISTS OF GREAT BRITAIN Publication Office: 54, Woodlands, London, N.W. 11 ¸ 1961 Society of Cosmetic Chemists of Great Britain VOL. XlI OCTOBER 1961 7 MECHANISMS OF DETERGENCY D. G. STEVENSON, Ph.D., A.R.I.C.* A lecture delivered before the Society on 16th January 19•1. A number of in•livklual mechanisms contribute to the overall •letergency l•rocess, e.g. l•re•erenfial wetting, solubilisation, comlflex formation or l•ene- tration anal mechanical action. The extent to which each contributes del•en•ls on the nature of the substrate, soiling matter anal aqueous l•hase. In a•klition, certain sl•ecific secon•lary effects, such as Sl•ontaneous emulsification anal osmotic swelling may coml•licate the l•rocess. The stability of the final disl•ersion is a function of the interfacial tension of the final oil l•hase, the l•hase con•lition of the soiling matter, anal the magnitmle of the •1ouble layer zeta l•otential. OwR TH• past 25 years the understanding of the detergency process has progressed slowly compared with the technological advances in detergent products nevertheless a fairly clear qualitative picture has even evolved. In this review, the system is considered primarily from the textile point of view. The situation on skin surface will be similar, although possibly somewhat simpler. The particular class of compound to which all the usual detergents belong is that of the paraffin chain salt or condensation product. By virtue of the distinct and separate oleophilic and hydrophilic portions of the molecule such compounds adsorb strongly at all interfaces, lowering the interfacial tension and modifying the double layer zeta potential. The other property of particular importance in this context is the *Atomic Weapons Research Establishment, Aldermaston, Berks. 353
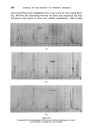
354 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ability of such molecules to aggregate into "micelies" above certain characteristic critical concentrations. The chemical and physical nature of dirt or soiling matter varies widely and it is not possible to define in any simple fashion. Brown • has provided data on the extractable matter in a range of domestic textiles (Table 1). These may be taken to illustrate a broad principle rather than anything precise and constant. Table 1 Composition of Soiling Matter Free fatty acids ............ 31.4% Triglycerides of fatty acids .......... 29.2% Saturated and unsaturated fatty alcohols and cholesterol 15.3% Saturated and unsaturated hydrocarbons ...... 21.0% Total extractable = 0.75% of dry weight of fabrics The high percentage of polar material (75.9%) should be borne in mind since this has a marked bearing on the mechanism of detergency to be discussed later. In addition to the oily matter, dirt includes insoluble pigment matter, e.g. carbon, silicates, etc., which is difficult to estimate and little precise work appears to have been done. Finally, traces of water soluble material are present, which, while not constituting a problem in themselves, modify the detergent process to a significant extent. WETTING OUT As a preliminary to the true detergency reaction, the detergent solution must come into intimate contact with the surfaces to be cleaned, thereby displacing all air from the surface, and in the case of textiles from the •a• Al• I•TE I SOLID Figur• 1 interstices of the fabric and yarns. When a liquid is in contact with a solid surface (Fig. 1) the angie of contact 0 is defined by the magnitude of the relevant surface or interfacial tensions, and by resolving parallel with the surface one finds yas -- 7ws yas = yws q- yaw Cos O, or Cos 0 -- yaw
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)