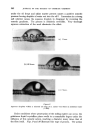
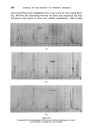
CURRENT TRENDS IN COSMETIC PRESSURE PACKS 409 REFERENCES _Financial Times, London (lst June 1961) CSMA Bulletin No. 164-61 (Chemical Specialties Manufacturers Association, New York) CSMA Bulletin No. 157-60 (Chemical Specialties Manufacturers Association, New York) Drug and Cosmetic Ind. 88 277 (1961) Schimmel Briefs 310 (January 1961) U.S. Pat. 2,953,498 U.S. Pat. 2,919,279 U.S. Pat. 2,948,656 U.S. Pat. 2,956,927 U.S. Pat. 2,957,838 tarf•merie und Kosmetik 42 8 (February 1961) Chemist and Druggist 175 8 (27th May 1961) •4tarfiimerie und Kosmetik 42 153 (April 1961) Geary, D.C. Technical Service Department Report (1960) (Union Carbide Chemicals Company, New York) Soap, Perfumery • Cosmetics 34 277 (1961) Chemist and Druggist 175 542 (1961) Chemist and Druggist 174 569 (1960) Soap Chem. Specialties 36 127 (November 1960) Modern Packaging 34 87 (December 1960) West, R.D. Private communication (1960) DISCUSSION Introduction by the lecturer I have recently come across 1960 production figures which disagree with the ones cited.* Cosmetic and toilet goods are thought to account only for 10% of the total, and it is also stated that 13 different products are concerned. I have referred to the piston-type container •*. I now know that it is not correct to state that the product and propellant are completely separate. With the particular hair cream, the product is in fact saturated with nitrogen, which has by-passed the piston. MR. C. BLOOM: (1) I agree entirely with the lecturer that the toothpaste pressure pack is an example where the packaging principles involved have no real advantages over existing packs and I would say that this sort of development does harm to the aerosol industry. I do, however, believe that hair cream pressure packs have the same disadvantages and it seems to me that the newer non-pressurized forms of packaging of these products are admirable. But no doubt there are people here today who can reply to this one. Are there possible cost and convenience advantages for such a pressure pack ? *Mfg. Chemist 32 282 (1961)
410 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (2) Can the lecturer please expand on the method of increasing the .amount of powder in a powder aerosol spray ? THE LECTURER: (1) I do not think that the nitrogen-filled dental cream pack harms industry in any way. Bad products which break down and deteriorate harm the industry, but if a product is formulated correctly, it cannot possibly harm us. In normal circumstances, hair cream is taken from a jar and usually •an excessive amount is withdrawn, but with a compressed gas propelled product, and with stream ejection, the amount of product extruded can be better controlled. When a material is too thin to be packed in a tube, •and too viscous to be packed in a bottle, a nitrogen pack is ideal. (2) West bases his formulations on specific gravities and specific volume. He commences with a given volume of propellant, and a given percentage •of powder and then bulks his powder with some material such as Snowfloss or Santocel 54. Only a further small amount of powder to fill the remaining volume is needed and in theory the two powders remain suspended indefini- tely. I have, however, not succeeded in verifying these results. West, in conjunction with an American valve manufacturer, has laid down a specification for a special powder valve with large passages which are few in number in order to aid the rapid exit of powder, and to avoid dogging. MR. H. S. FORBES: (1) Can the valve be designed to act as a safety •device ? (2) What is the position concerning hydrocarbon propellants ? (Cost •vs. inflammability vs. corrosion). THE LECTURER: (1) I presume you wish to prevent explosions in the event of excessive pressures. The valve must be sufficiently secure to prevent the contents from escaping, and it must also be able to pass the hot water test. Safety devices of a sort are being tested in the United States, where they are necessary because of the number of incinerators, and the number of explosions which do occur. A fusible plug is being considered for this purpose. The plug will melt at a temperature below the explosion limit. If the container were to be subjected to a temperature which is not extreme .enough to explode the container, but of sufficient temperature to melt the plug, the latter could be activated without necessity. Nevertheless, this will probably be employed in the United States. (2) Hydrocarbon propellants are cheaper than Arcton propellants, and more expensive than the compressed propellants. Butane, isobutane and propane are included in this class and are somewhat odorous this aspect
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)