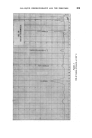
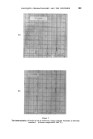
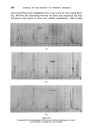
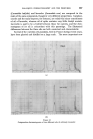
372 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (b) In research for the isolation and identification of unknown compounds present in essential oils for the separation of "difficult" isomers in the pure state, especially in studies of the relationships between odour and structure for studying reactions in experiments to develop new perfumery ingre- dients or to improve processes for making well-known ingredients for investigating the limitations of chemical methods of analysis and the causes of observed inaccuracies in them. (c) In manufacturing for following and controlling the courses of reactions and fractional distillations. Other uses may have been devised of which I have no knoMedge, but even so the list is impressive enough. It will doubtless be added to in the future. CHOICE OF WORKING CONDITIONS In perfumery work, particularly when essential oils are studied, it is important first of all to learn how to make the best use of available equip- ment. Perfumers who pile on the samples without thought for the optimum capacity of their instrument must take much of the blame for their own disappointments. In the long run, time is saved when favourable conditions for achieving required separations are known. Essential oils are among the most difficult of mixtures to which gas-liquid chromatography has yet been applied. They have very many components, which may vary over an extensive range of concentration, vapour pressure and chemical composition, and quite minor components can contribute significantly to the complete odour. Fig. 1 is a chromatogram of components of oil of Ceylon citronella, eluted in 3 hours at 100 ø C. Geraniol makes up more than one quarter of the total, while the smallest peaks represent only fractional-percentage constituents. The first-eluted compounds have reten- tion times of only a few minutes, but other substances (not all shown on the illustration) have retention times of up to 14 hours. The diversity of possible chemical compositions gives rise to even more problems. Substances occurring in essential oils all contain carbon and hydrogen. The majority contain oxygen, a few include nitrogen, and occasionally sulphur is present. Hydrocarbons, ethers, alcohols, phenols, esters, aldehydes, ketones and lactones are commonly found. Structures include unbranched and branched chains, alicydic, aromatic and heterocyclic rings, and some more complex bridged rings.. Isomeric compounds often occur together, including stereoisomers. Resolutions of related compounds which form simple sequences rarely overtax the skill of the gas-chroma- tographer. Fig. 1 shows the degree of separation obtainable with three
GAS-LIQUID CHROMATOGRAPHY AND THE PERFUMER 373
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)