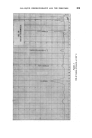
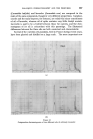
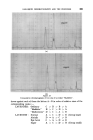
THE DETERMINATION OF WATER IN SHAMPOOS BY DISTILLATION 3994 there was considerable initial foam with the glycerine. However, after the first portion of water had distilled the foam subsided and finally ceased. In both cases, the initial slurry in the flask cleared and gave a single phase during the distillation. When further water had distilled, the glycerine separated as a liquid phase which gradually darkened, and which was still_ evolving water after 3« hours. On the other hand, in the test with the oleic acid a fine powder separated which rapidly coagulated on the walls of the flask. The results and the observations of conditions in the flasks are given_ in Fig. 1. (The time scale has been made logarithmic for convenience.) Other shampoos, particularly those based on the ether sulphates and incorporating alkanolamide foam boosters and stabilizers, are sometimes difficult to analyse due to the excessive and very stable foam even when oleic acid is used. It was found that the addition of a quaternary ammonium compound precipitated some of the anionic detergent and foaming could be eliminated even when the addition was less than stoichiometric. One such shampoo which proved very difficult to analyse even with the addition of oleic acid, responded perfectly to this treatment, the distillation of water being complete within ten minutes of starting to heat the flask. On the other hand, in the absence of the quaternary, three out of four attempts at the distillation resulted in the contents of the flask foaming IS . Foam/rig S • • 40 •o Mino• t•m commen•ment at Heating Figure • Sample A B Oleic Acid .. ml 10 10 Quaternary .. g 1.0 nil Xylene .. ml 105 105
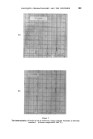
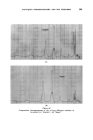
-400 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS •over into the condenser despite every care and attention. These two analyses are compared in Fig. 2. The quaternary ammonium compound used in these tests was commercial lauryl pyridinium chloride (Dehyquart C) but others were equally as effective. For use, the crystals were alehydrated by heating in oleic acid to 170•180øC at which temperature they dissolved. On cooling, the bulk of the quaternary crystallized out. The viscosity of this slurry was reduced by the addition -.of about half its volume of xylene. It then proved to be a convenient reagent, allowing the addition of the oleic acid and the anhydrous quaternary compound at the same time. DISCUSSION The results presented in Fig. 1 show that, not only is oleic acid a better reagent than glycerine for reducing the foaming, but also that continuous heating decomposes glycerine with the evolution of water. Since the -glycerine had been heated until the liquid temperature had been constant for five minutes (279øC at 630 ram) this additional water must have come from the pyrolysis of the glycerine and/or the shampoo components. That the glycerine was at least partly responsible was confirmed by the odour of acrolein from the black glycerine syrup left in the flask. When the oleic acid alone gave adequate control of the distillation, the use of the quaternary ammonium compound gave but little benefit (Fig. 1). -When the oleic acid alone gave insufficient control of the foam, the preci- pitation of part of the artionic detergent by the quaternary ammonium compound gave excellent control (Fig. 2). CONCLUSION , The addition of an anhydrous quaternary' ammonium compound has been shown t6 control the foaming of shampoos during distillation and thus .allows more rapid and trouble-free determination of the water content. (Received ß 17th May 1961)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)