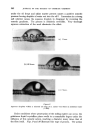
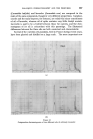
MECHANISMS OF DETERGENCY 3(•1 NON-IONIC DETERGENT ACTION The behaviour of non-ionic detergents differs in that the penetration and solubilising power appears to be even greater than their ionic counter- parts. This may be expected from their non-ionic nature which renders them appreciably soluble in hydrocarbons as well as in water. Spontaneous emulsification of oil blends is replaced by direct penetration and solubiliza- tion, the detergent acting as a co-solvent. Small 5t• drops of, for example, a 25% oleic acid in mineral oil will be observed one moment and then suddenly disappears in a "puff". Larger droplets (20t•) leave the surface under the action of their own buoyancy (Fig. 7) before disappearing in a similar manner. (The surface tension of the drop shown is estimated to (a) (b) Figure 7 Removal of a 3: I mixture of Nujol and oleic acid from wool by a 0-2% non-ionic detergent solution ( x 300). (a) 30 sec. (b) 3• min. be as low as l(k a dyne/cm.) It appears that in this system the detergent being oil soluble penetrates the oil phase, and finally renders the oil mixture water soluble. The finite time of consolution is due to diffusion across the interface, thus the time required for disappearance is proportional to the volume/surface area ratio (which is in turn proportional to the radius). OSMOTIC EFFECTS In most practical examples, soiled surfaces are contaminated with a finite amount of water soluble matter in addition to fatty and pigment soiling. On immersion, conditions exist for osmotic flow of water through the surface and through the oil. Fig. 8 illustrates the simplest form of the action. In this example the wool was well impregnated with the sweat (suint) salts of the sheep although all the fatty matter had been extracted. On immersion, the uncovered areas of the fibre admit water which swells the fibre in the usual manner. The water then penetrates along the fibre
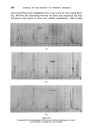
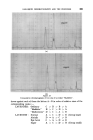
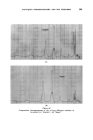
362 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS under the oil drops and saline matter present causes a positive osmotic pressure forcing droplets of water out into the oil •. Immersion in a strong salt solution causes the aqueous droplets to disappear by reversing the osmotic gradients. The process is infinitely reversible. Very thorough aqueous extraction of the wool eliminates the effect. (a) 1 hour. (b) 48 hours. (c) 7 days. Figure 8 Aqueous droplets within a mineral oil drop on a saline wool fibre in distilled water ( X aO0). Under conditions where penetration of the soiling matter can occur, the gelatinous liquid crystalline phase swells to a remarkable degree under the influence of this osmotic action, reaching a diameter many times that of the fibre itself. Figs. 9 and 10 illustrate this type of process. The action
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)