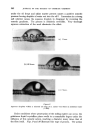
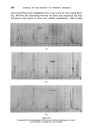
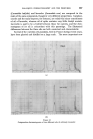
MECHANISMS OF I)ETERGENCY 355 Detergent molecules being surface active adsorb at the solution interfaces and the corresponding interfacial tensions are reduced (viz. yws and yaw) so that the third force (yas) is no longer balanced and the line of contact is pulled forward until a new contact angle is established. In practice, with most detergent solutions on common surfaces 7as yws -t- yaw thus 0 = 0 and wetting is complete. REMOVAL OF SOILING MATTER One of the early theories of detergency, attributed to Berzelius, stated that fatty material was removed by saponification caused by the free (hydrolytic) alkali in a soap solution. This is readily disproved by the efficiency of synthetic non-hydrolysing detergents which can act under neutral conditions. In 1937, Adam * published his work on the "rolling up" process which formed the first major milestone in the understanding of detergent action. The action is basically similar to wetting out except that the phases are exchanged (Fig. oe). Here again at the line of intersection between the solid surface and the oil/water interface, the angle of contact 0 is defined by resolving the forces parallel to the solid surface such that yos -• 7ws -- y and Cos 0 7ws -- 7os thus Cos y = yow The adsorption of detergent on to the solution interfaces again lowers the relevant interface tensions so that the unbalanced yos is able to constrict the base of the oil drop progressively as shown in Fig. oe. The equilibrium contact angle is a function of all the phases present. In general, the more polar the solid and oil the greater the contact angle. This is illustrated by taking a series 8 of filaments ranging from P.T.F.E., through polythene, terylene, nylon, wool and on to cotton. Likewise a pure hydrocarbon mineral oil is extremely difficult to remove from the less polar fibres, although blends with polar oils overcome this difficulty. In the non-polar systems the oil/solid interfacial free energy is so low that it remains below the figure attainable by most detergents at the aqueous/solid interface. One prime requisite for the rolling up process is that the fatty material shall be fluid. This is obvious from the nature of the process, and is illustrated by washing studies with waxy soiling matter, where one finds a threshold temperature for detergency at the melting point of the wax. In many practical examples it is found that the oily soiling matter rolls up to a complete sphere with a contact angle of 180 ø whereupon the oil becomes detached and floats away under its own buoyancy. If the detergent solution, however, is in motion, viscous drag will distort the
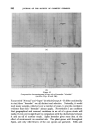
356 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS drops and remove them before the contact angle reaches its ultimate value. With very small oil drops (approaching 1/•) Brownian movement cannot be entirely ruled out as an assisting force. ß SOLID 905 e = It•0 • Figure 2 SOLUBILIZATION Under certain conditions, oils and oil soluble material are rendered soluble in aqueous media by the action of surface active agents. In the form first discovered it was shown that hydrocarbons such as heptane were incorporated into the interior of the detergent micelie and in effect appeared to "dissolve" in the hydrocarbon tail core of such micelies. McBain 5 considered this to be a promising explanation for the efficiency of detergents in removing oils. At the time, however, there were many argmnents against such an explanation. More recently a second type of solubilization has been defined whereby polar long chain, oil soluble matter (e.g. fatty acids and alcohols) are adsorbed into the "palisade" region of the micelie, to be aligned with the molecules of detergent. Finally, of course, both types can operate simultaneously, and the presence of one type of solute in such cases increases the solubility of the other. The amount of material which will dissolve in a detergent is of an order which would correspond to an appreciable proportion of the soiling matter usually present, providing the detergent is reasonably well above the critical miceliar concentration.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)