MECHANISMS OF DETERGENCY 357 Solubilization is a property of detergent micelles and commences only at the critical miceliar concentration. Detergent efficiency, however, rises steadily from far lower levels, and tends to level off at the critical micellar concentration. Commercial wash liquors often run very close to this value. As Preston 6 has concluded, this would appear to relegate solubilization to a rather minor role in detergency processes. This aspect has, however, been affected by the discovery of the pene- tration processes described below. It is possible that, particularly with polar fatty matter, so!ubilization may be a final state after intermediate processes. COMPLEX FORMATION Mixed Adsorbed Films It has been known for some time 7 that where an oil soluble surface active matehal is present in a system in addition to the water soluble detergent, far lower interfacial tensions are attained than would be possible with either component alone. Oil soluble surface active matehals include long chain polar compounds such as fatty alcohols, fatty acids, and indeed amines, mercaptans, etc. Such matehal may be present within the oil phase or "solubilised" in the aqueous phase. The benefit derived from blending a polar material into a non-polar mineral oil was recognised several years ago 8. By adding oleyl alcohol for example, the oil which formerly proved extremely resistant was easily removed. It may be shown by microscopical observation that a solution of sodium oleate (0-2%) will displace mineral oil from a wool fibre. If alkali, however, is added to the solution, the action is inhibited and the soap is then no more effective than an alkyl sulphate detergent. The efficiency of the plain sodium oleate solution is due to the hydrolytic fatty acid which serves as the oil soluble polar compound and facilitates the additional lowering of inter-facial tension (to less than 1 dyne/cm) to enable the mineral oil to be displaced. In the same manner as with polar solubilisation, the oil soluble polar com- ponent merges with the "palisade" of detergent molecules adsorbed at the solution interfaces and enhance the surface active effect. PENETRATION As early as 1941 Kling and Schwerdtner 9 described an interaction between oleic acid drops and a detergent, and concluded incorrectly that spontaneous emulsification was occurring. In the author's studies TM it was apparent that the type of action which Kling and Schwerdtner had observed was typical of a wide range of detergent--long chain polar com- pound (amphiphile) systems, and was in fact an interaction between the two species leading to a liquid crystalline phase. When a crystal of
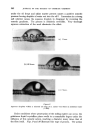
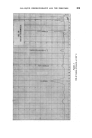
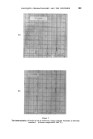
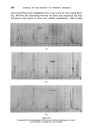
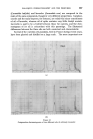
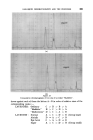
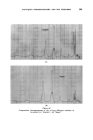
358 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS cholesterol, Fig. $, or of lauric acid, Fig. 4, is immersed in sodium laurate, for example, a halo forms round the material which comprises a mass of myelinic figures. This material is gelatinous, as shown in Fig. 4 in which the original solid drop of lauric acid has become eroded and separated from the fibre, but remains anchored by the complex mass. trigure $ Myelinic figures (cholesterol-1% sodium laurate ß 20 min ß x 100). trigur• ,l Lauric acid undergoing penetration by 0-5% sodium laurate (X 100, 5 mins, 25øC.). More recently, Lawrence n has obtained phase diagrams and much other data which explain in more precise terms most of the earlier observations. Fig. $ shows a typical phase diagram for a water-detergent-amphiphile system: L, is the normal soap solution containing solubilised amphiphile
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)