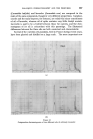
MECHANISMS OF DETERGENCY 359 (fatty acid or alcohol). L2 is the converse, where the amphiphile is the solvent phase and water the solute. In the lower region L• and L•, the two liquids exist together and would readily emulsify into a conventional emulsion. The region LC is that of the viscous liquid crystalline material. When amphiphilic material is immersed in a detergent solution the com- position of the material moves along a tie line, e.g. A--Z and as the material swells, a membrane of liquid crystalline material is formed at the surface of the amphiphile. This membrane slowly dissolves into the detergent (L•). Lawrence postulates that as the adsorbed soap at the surface is in a condensed state the effective concentration Z is much higher than in the bulk solution. The tie line A--Z will thus be well removed from the AW axis. SOLID L•.+ L L• L! Al'lP141PHILE Figure $ The penetration of polar materials such as alcohols and acids proceeds down to a well-defined threshold temperature, well below the melting point of the compound (10--40øC lower, depending on the detergent). Further- more, the formation of eutectic mixtures of amphiphiles is reflected by a corresponding lowering of the penetration threshold temperature. Whereas the simple "rolling up" mechanism of detergency relies on the fatty matter
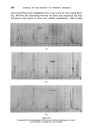
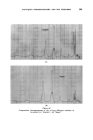
360 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS being fluid, penetration by detergent will remove such matter well below the melting point. At higher temperatures, the liquid crystalline phase becomes increasingly soluble and an upper consolute temperature is observed. In some cases, however, this is above 100øC. Microscopical studies of detergency process lend strong support to the view that in the large majority of practical cases penetration plays a major role in detergency. The pre- sence of amphiphilic material is certainly well illustrated by Brown's data given above. However, such microscopical studies do not support Lawrence's assertion that cryoscopic forces are the only mechanism in detergency. SPONTANEOUS EMULSIFICATION Where the soiling matter is not entirely amphiphilic but contains hydro- carbons, esters, and less surface active material of an oleophilic nature in admixture, spontaneous emulsification occurs on contact with a detergent solution. Drops of oily material greater than 15-20t• diameter tend to develop gelatinous "atmospheres" which contain a dispersion of droplets in the 1-5t• range. This process is distinct from the spontaneous emulsi- fication of oil blends containing free fatty acid when added to alkal/ne solutions. Observed under the microscope, the latter appears as a vigorous reaction when the surface of the oil phase violently convulses as n eutralisa- tion proceeds. The former is a quiescent process where the droplets merely "grow" in the gelatinous atmosphere. The quiescent emulsification may be explained readily by reference to Lawrence's phase diagram (Fig. $). Mineral oil, etc., will be readily soluble in the amphiphile and in the amphi- phile-rich liquid L•,. It is probable that the solubility in the liquid crystalline phase will be somewhat less, and very much less in the detergent solution L1. Thus as the amphiphile mixture is penetrated and the phase change occurs, the non-polar oil is precipitated. This action is clearly illustrated in Fig. 6, in which the precipitated oil drops are held within the smaller complex atmosphere. Figure Removal and emulsification of a large drop of natural soil from wool, in 0-2% sodium oleate (2 min x 150).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)