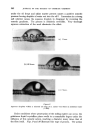
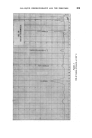
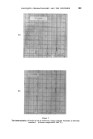
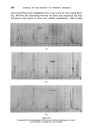
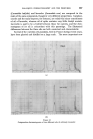
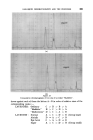
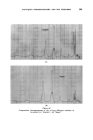
396 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Minor differences exist between these, and chromatograms of the correspond- ing steam distilled oils (Figs. 11 and 15), but "formula" of Groups A--D are unchanged. Flower chromatograms such as these might serve as a guide to plant breeders as well as to perfumers, and plant geneticists could no doubt make profitable use of them also. ILLUSTRATIVE CHROMATOGRAMS All chromatograms reproduced in the illustrative slides were made with the standard "Pye Argon Chromatograph" and the two integrams were done with the "Pye" integrating amplifier. The column was 120 cm long, and, where not otherwise specified, it was packed with "Celite", 100-110 mesh, containing 10% w/w of "Reoplex 100" (polypropylene glycol sebacate). Column temperature was 70 ø C except where another temperature is stated. The carrier gas was Argon. ACKNOWLEDGMENTS I should like to express my thanks to Lautier Fils, S.A. who were so good as to enable me to collect authentic samples of oils of the various strains of lavender and lavendin from distilleries in S.E. France and to Sr. Ram6n Bordas who kindly made it possible for me to visit all of the chief production areas of spike lavender oil in Spain. (Received: loth March 1981) •DISCUSSION MR. H. B. HEATH: Has the lecturer any information on the chromato- graphic picture of the one or two species of lavender grown in England, as compared with the French species and varieties ? THE LECTURER: I have not examined oils of English origin under similar working conditions. DR. H. W. HIBBOTT: The significant differences on the chromatograms seem small. Would a perfumer detect the differences which are regarded as significant ? THE LECTURER: Differences revealed by the chromatograms of oil of spike certainly seem small, but an experienced perfumer can distinguish between those particular oils. They may also vary in content of less volatile substances not shown in the illustrations, but even if they were similar in this respect, I am sure that the perfumer would have few doubts about their individuality.
THE DETERMINATION OF WATER IN SHAMPOOS BY DISTILLATION 397 MR. L. G. TOWnRS: When chromatographing lavender oils at relatively low column temperatures in order to resolve as completely as possible the components of shorter retention times, what do you consider to be the minimum column temperature below which sample preheating becomes necessary ? THE LECTURER: No significant loss of performance was observed when samples of up to 100 #g were put straight on to a column at 70 ø C, though at lower temperatures a preheater may be needed. The answer will probably depend upon the size of sample required for the instrument used, but in any case no harm could be caused by a sample preheater at, say,, 100 ø C. THE DETERMINATION OF WATER IN SHAMPOOS' BY DISTILLATION G. E. MAPSTONE, M.Sc., Ph.D., F.R.I.C. Some shampoos, etc., foam excessively' on distillation even after the addition of oleic acid. The addition of a quaternary ammonium compound in such cases allows the ready distillation of the water. INTRODUCTION THE DETERMINATION of water in shampoos and similar products, by distil- lation (Dean and Stark Method) presents a special problem in that such products normally contain materials of high foaming power, and frequently also a foam stabilizing agent. • Gentle spot heating of the flask just below ihe liquid surface can often control the foaming by circulating the flask contents and will, at all times, cause a reduction in the amount of foam present. This technique, however, requires the undivided attention of the operator and, with Well foaming materials is frequently inadequate. Even when the foaming is kept under control, the solid detergents can set as a cake, as the water distils. If due care is not taken this cake can adhere to the bottom of the flask where it ca.n .either occlude water or .char. This can lead to low results by the failure of all the water to distil, or to high• r•esults due to the water formed by the decomposition. Occasionally, the detergent precipitates as a fine powder which can physically stabilize the foam but, if it does happen, it is usually transient due to the tendency of the powder to agglomerate. The addition of a non-volatile solvent that will dissolve the anhydrous detergents can reduce, and frequently overcome, these problems. Two such *Dermacult S.A, (Pty.) Ltd., Johannesburg, South Africa.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)