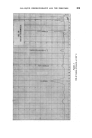
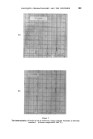
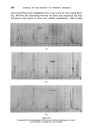
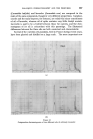
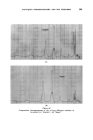
378 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS successive chromatograms on the same polar stationary phase at varying temperatures. I believe that many workers with essential oils choose column temperatures too high for adequate resolution of any but the least volatile components. (Some published chromatograms have even shown clear evidence of partial thermal decomposition, indicated by a general lift of the base-line.) This predilection for high temperatures, perhaps due to impatience, may well explain why the magnitude of the effect which I propose to describe seems hitherto to have been overlooked. At 120 ø C and above, the first-eluted fractions of essential oils tend to produce an unsightly jumble of concurrent peaks. Good resolution is hardly possible until the column temperature is dropped below 100 ø C. During a study of the early fractions of oils of lavendin on p.p.s. at 70 ø C, I observed a peak where no peak had occurred in chromatograms at 100 ø C. It proved to be camphor, which overlaps with linalol at 100 ø C and more. It was easy to demonstrate the increasing separation of camphor from linalol with successive reductions of temperature (Fig. 4). Another chromatogram of oil of geranium on p.p.s. at 70 ø C (Fig. 6) revealed a clear separation of linalol from isomenthone, which is not possible on the same column at 100 ø C (Fig. oe). "%' • •'!% 2 •:. Z .:: X:.'. :' :::' :: 7 -. Figure 8 Separation of linalol from isomenthone by reduction of column temperature (see also Fig. oea). Peak resolution* for linalol-camphor was 0 at 120 ø C, 1.4 at 00 ø C and 3.0 at 70 ø C. For linalol-isomenthone, the figures were 0 at 100 ø C and *Expressed as twice the difference between retention times, divided by the sum of the two peak widths, measured in time units.
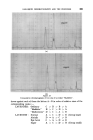
GAS-LIQUID CHROMATOGRAPHY AND THE PERFUMER 379 2.4 at 70 ø C. The effect is not confined to dissimilar pairs like an alcohol and a ketone. On the same type of column, the resolution of geraniol/nerol increased from 2.6 at 110øC to 4.0 at 70 ø C that of citral-a/citral-b from 1-9 at 110 ø C to 2.9 at 100 ø C, and that of terpineol-a/terpineol-b from 3.0 at 110 ø C to 5.5 at 70 ø C. This appears to be a retention phenomenon rather than a variation in column-efficiency, so it can work equally in reverse. For example, the peaks of nerol/citronellol are almost coincident at 70 ø C but not at 100 ø C, and bornyl acetate/linalyl acetate, separated at 100 ø C, overlap at 70 ø C. I recommend the method to the attention of essential oil chemists as worthy of trial, particularly because it demands only the adjustment of one of the most flexible variables on any instrument--the column tempera- ture. A better understanding of this influence of temperature on resolutions will certainly contribute considerably towards achievement of desirable "optimum" working conditions. GAS-LIQUID CHROMATOGRAPHY AND QUANTITATIVE ANALYSIS Perfumers and manufacturers of perfumery ingredients are naturally interested to find out as much as possible about the composition of their raw materials. Several chemical methods of quantitative analysis have been devised, but few are really selective. They determine functional groups only. Many perfumery materials contain more than one compound with the same functional group. Their sum total may be found, but varia- tions in their relative proportions (which may greatly affect the perfumer's assessment) cannot easily be measured. For instance, the "ester value after acetylation" has little meaning for evaluating a "citronellol", which may contain a large proportion of geraniol and dihydrocitronellol, or alternatively for a geranium oil containing geraniol, citronellol, linalol and minor amounts of other alcohols. Gas-liquid chromatography offers the possibility of separating any chosen component of a mixture, and consequently of determining the quantity present. In theory, physical isolation followed by weighing is possible, but in practice it is difficult. Preparative-scale gas-liquid chro- matography is generally less efficient than analytical-scale gas-liquid chromatography and it is not easy to trap all of the eluted material. The more usual method is to introduce a pure reference compound (known as the "internal standard") into the sample at a known concentra- tion, and then to compare detector responses to it, and to the ingredient which is to be determined. It is important that this ingredient be repre- sented by an isolated, homogeneous peak, and that the peak of the internal standard occupy a position where the base-line is free from any other peak. Fig. 6 shows how n-butyl benzoate may be used to determine carvone in
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)