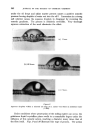
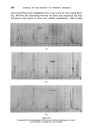
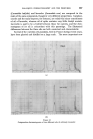
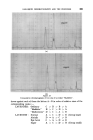
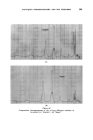
MECHANISMS OF DETERGENCY 369 imposed on the surface. This assists the removal and coarse emulsification of the oil. The specific action of foam is most clearly demonstrated in Fig. 14 where in the presence of a cationic surface active agent, the oil is actually spread further over a glass surface by the foam. CONCLUSION From the foregoing it will be appreciated that there are a number of physical and chemical processes which contribute to the removal of dirt from surfaces. The extent to which each operates depends upon the composition of all three major components of the detergent-surface-dirt system. Other factors not discussed here also operate under certain conditions. The subject has been treated from a fundamental viewpoint. In prac- tical detergency there ar• other factors which' must be l•orne in mind, e.g. buffering action and stability towards calcium'and magnesium ions. Atten- tion to such details will ensure that the detergent composition will act in the most efficient manner, but will not affect the mechanism of the process. While the broad principles by which detergents act are now becoming clearer there are innumerable details to be filled in. There is unfortunately a lack of incentive 'to elucidate the system from a fundamental viewpoint since the 'major developments in practice have usually 'been achieved by empirical 'experimentation the'full understanding of the process has followed at a slower pace. ACKNOWLEDGMENTS Figs. 3, 4, 6-10 have appeared previously in J. Textile Inst. 44 T12 1953). Figs. 12-14 have appeared previously in J. Soc, Dyers Colourists 68 57 (1952). , (Received: 27th March 1961) REFERENCES Brown, C. B. Research, London 1 46 (1947) Adam, N. K. J. Soc. Dyers Colourists 53 121 (1937) Harker, R. P. J. Te vtile Inst. 50 T189 (1959) Stewart, J. C., and Whew•ell, C.S. Textile Research J. 30 903 (1960) Klevens, H.B. Chem. Revs. 47 1 (1950) McBain, J.W. Advances in Colloid Science (1942) (Interscience Publishers, Inc., New York) Preston, W. C. J. Phys.. Colloid Chern. 52 84 (1948) Schulman, J. H., and Cockbain, E.G. Trans. Faraday Soc. 36 651 (1940) Speakman, J. B., and Chamberlain, N. H'. Trans. Faraday Soc. 29 358 (1933) Kling, W., and Schwerdtner, H. Melliand Textilber. 22 21 (1941)
370 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Stevenson, D. G. The Mechanism of Detergent Action (1951) (Ph.D. Thesis, London University) J. Textile Inst. 4,4, T12 (1953) Lawrence, A. S.C. Nature 183 1491 (1959) Faraday Soc. Discussions 25 51 (1958) Stevenson, D. G. J. Textile Inst. 4,2 T194 (1951) Stanley, J. J. Phys. Chem. 58 533 (1954) Kruyt, H.R. Colloid Science 1 Ch. 6 (1952) (Elsevier, London) McBain, J. W., Colloid Science 21 (1950) (Heath, Boston) Sawyer, W. M., and Fawkes, F.M. J. Phys. Chem. 62 159 (1958) Niven, W.W. Fundamentals of Detergency 4 (1950) (Reinhold, New York) Wark, E. E., and Wark I.W. Nature 14,3 856 (1939) Stevenson, D. G. J. Soc. Dyers Colourists 68 57 (1952) GAS-LIQUID CHROMATOGRAPHY AND THE PERFUMER D. HOLNESS, B.A.* A lecture delivered before the Society on 2$rd February 1961. Gas-liqukl chromatogral•hy is an efficient sel•aration technique which can simlflibj the l•er•umer's stmlies of coml•lex raw materials. Its alrea•ly numerous al•l•lications are liste•l, anal the nee•l for careful choice of working con•li•ions is stresse•l. Examlfles are given of quantitative analyses aml of klen•ifications of essential oils of similar tyl•es. THE TERM "chromatography" covers a group of closely related separation techniques, all based on partition between two phases, one fixed and one moving. Chromatographic methods are outstanding in efficiency, and have the additional merit of working under conditions which permit the safe handling of many relatively unstable compounds. Gas-liquid chroma- tography, distinguished by having a gas as mobile phase and a liquid as stationary phase, is applicable to substances with appreciable vapour pressures at moderate temperatures. Perfumers frequently work with mixtures of uncertain composition (notably the essential oils) and their raw materials have odours, a property which implies some degree of volatility. Consequently chromatography has obvious applications in this field, with gas-liquid chromatography as the method of choice. The value of gas-liquid chromatography to the perfumer lies mainly in its ability to simplify his studies of mixtures. It is by no means the answer to all his problems, nor is it a sort of "artificial *Proprietary Perfumes, Ltd., London, S.E.1.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)