THE DETERMINATION OF WATER IN SHAMPOOS BY DISTILLATION 397 MR. L. G. TOWnRS: When chromatographing lavender oils at relatively low column temperatures in order to resolve as completely as possible the components of shorter retention times, what do you consider to be the minimum column temperature below which sample preheating becomes necessary ? THE LECTURER: No significant loss of performance was observed when samples of up to 100 #g were put straight on to a column at 70 ø C, though at lower temperatures a preheater may be needed. The answer will probably depend upon the size of sample required for the instrument used, but in any case no harm could be caused by a sample preheater at, say,, 100 ø C. THE DETERMINATION OF WATER IN SHAMPOOS' BY DISTILLATION G. E. MAPSTONE, M.Sc., Ph.D., F.R.I.C. Some shampoos, etc., foam excessively' on distillation even after the addition of oleic acid. The addition of a quaternary ammonium compound in such cases allows the ready distillation of the water. INTRODUCTION THE DETERMINATION of water in shampoos and similar products, by distil- lation (Dean and Stark Method) presents a special problem in that such products normally contain materials of high foaming power, and frequently also a foam stabilizing agent. • Gentle spot heating of the flask just below ihe liquid surface can often control the foaming by circulating the flask contents and will, at all times, cause a reduction in the amount of foam present. This technique, however, requires the undivided attention of the operator and, with Well foaming materials is frequently inadequate. Even when the foaming is kept under control, the solid detergents can set as a cake, as the water distils. If due care is not taken this cake can adhere to the bottom of the flask where it ca.n .either occlude water or .char. This can lead to low results by the failure of all the water to distil, or to high• r•esults due to the water formed by the decomposition. Occasionally, the detergent precipitates as a fine powder which can physically stabilize the foam but, if it does happen, it is usually transient due to the tendency of the powder to agglomerate. The addition of a non-volatile solvent that will dissolve the anhydrous detergents can reduce, and frequently overcome, these problems. Two such *Dermacult S.A, (Pty.) Ltd., Johannesburg, South Africa.
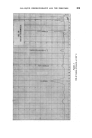
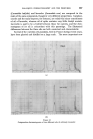
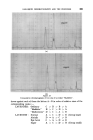
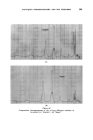
398 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS solvents that have found favour are g!ycerine and oleic acid, the latter being preferred by the American Oil Chemists Society (e.g.A.O.C.S. Methods Ca 2b-45 for soaps and F la44 for sulphated and sulphonated oils). In this communication a further improvement is reported which allows the ready analysis of very seriously foaming shampoos, etc. EXPERIMENTAL To compare the effectiveness of oleic acid and g!ycerine for control of foaming, the water content of a shampoo was determined using each in turn. Whereas there was no foam with this shampoo when using the oleic acid, /$ o 5 IO 20 50 Mit•ute, Prom Commencement Figure 1 Sample A B C Shampoo .. gm 20 20 20 Oleic Acid .. ml 10 10 nil Quaternary .. g 1 nil nil Glycerine .. g nil nil 30 Xylene .. ml 100 100 100 /00
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)