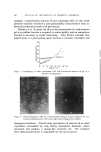
PYRIDOXINE-3,4-DIACYLATES IN COSMETICS 353 Group D: Standard Diet palmitate Group E: Standard Diet dipalmitate Group F: Standard Diet dipalmitate q- 7 mg./kg./day of pyridoxine-di- 70 mg./kg./day of pyridoxine- 700 mg./kg./day of pyridoxine- Groups A, B, and C were control groups. The weights of the rats were recorded daily for six months, and the growth rate is shown in Fig. 1. When pyridoxine-3,4-dipalmitate was orally administered daily to rats (in an amount of •000 the oral LD:•0 for mice) for six 340' Croup 4D 300, • • .•- ._ •..•" • 250- / •/' / -'-• ..... • •'• • 9-•./'" .• ..... • 200 • • •//'.• ..... • 20 • 60 80 100 120 l• 160 Figure 1.•Change in body weight of rats fed with various amount of pyridoxine-3,4-di- palmitate months, the increase of the animal's weight was greater than when the equivalent amount of pyridoxine hydrochloride was administered. All rats used in this test survived during the test and were killed at the end of the test for observation of organs. No harmful effect of pyridoxine- 3,4-dipalmitate was observed in organs at autopsy. Percutaneous Absorption (8) Rabbits weighing from 2 to 3 kg. were used for this test. An area of •5 X 15 cm. 2 on the abdomens of the rabbits was clipped, and the hair was completely removed with a depilatory. After 24 hours, 6 g. of hydrophilic ointment containing one of the pyridoxine derivatives was
354 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS applied after ascertaining that there was no inflammation on the part to be tested. Blood samples were collected from the ear lobe by in- travenous puncture and assayed for vitamin B0 content. The composition of the hydrophilic ointment is as follows: Isopropyl myristate 1 g. Cetanol 0.25 g. Stearic acid 0.4 g. Paraffin wax 1 g. NIKKOL BL-9* 0.2 g. NIKKOL BC-5t 0.15 g. Mineral oil 1.35 g. 10% Triethanolamine solution 1 ml. Pyridoxine derivative 1 • Water to make 1 • g. In the preparation of the ointment containing pyridoxine hydro- chloride, the triethanolamine solution was omitted from the above formulation. The vitamin B6 content in blood was determined as follows: Standard curve: In the case of pyridoxine hydrochloride and of pyridoxine-3,4-dibutyrate, 1 ml. of an aqueous sample solution of known concentration (about 0.5 m/mi.) was acidified with 6 mi. of 15% H2SO4, and the solution was heated at 100øC for one hour. After cooling, the pH of the solution was adjusted to 5.4, and the volume made up to 50 ml. with water. Samples of this solution (0.25, 0.5, 1 and 2 mi.) were pipetted into test tubes and diluted with water to 2.5 mi. After adding 2.5 mi. of culture medium and steriliz- ing at 100øC for 15 minutes, 1 drop of preincubated suspension of Saccaromyces Carlsbergensis culture was added, and the solution was incubated at 30 øC for 20 hours. Absorbancy was then measured at 610 mu, and a standard curve was constructed. For the construction of the standard curve for pyridoxine-3,4- dioctanoate, 1 mi. samples of ethanolic solutions were hydrolyzed as mentioned above. Pyridoxine assay in blood: A mixture of 1 g. of blood and 6 mi. of 15% H2SO4 was heated at 100 øC for one hour. After cooling, the pH of the solution was adjusted to 5.4, and the liquid was centrifuged to remove blood pigments. The supernatant solution was separated, and its volume was adjusted to 50 mi. with water. The microbio- * Polyoxyethylene lauryl alcohol ether. Nikko Chemicals Co., 1, 1 chome, Nihonbashi Bakurocho, Chiyoda-ku, Tokyo. t Polyoxyethylene cetyl alcohol ether. Nikko Chemicals Co.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)