Reaction products of dihydroxyacetone 31 spins/g range except for arginine which gave a lower value. Two of the melanoidins obtained with non-alpha aminoacids have a slightly higher linewidth and a stronger signal. II. REACTIONS CARRIED OUT AT 37øC In order to better investigate the possible reactions occurring actually in the Stratum Corncure, a few experiments have also been made at 37øC with the same starting con- centrations of DHA and aminoacids in water: 0.1 mole of each reactant (0-05 for Lys) in 25 mi. Only glycine and lysine did produce significant amounts of pigments after 24 h (Table V). Arginine reacted very poorly, as could be expected in view of the low yield obtained at 100øC. But what is more surprising is that serine and alaninc which react readily with DHA at higher temperature, are almost inert at 37øC. It should be noted, however, that these two aminoacids were not entirely soluble at this temperature in the rather concentrated media used. Table V. Melanoidins formed by reaction of DHA with aminoacids at 37øC Duration U.V. absorption Spins/g Aminoacid (h) •o Yield of 0'01 • water sol. (x 10 -•8) GIy 10 1.2 1'15 at 320 nm 2-5 Gly 24 1-7 1.40 at 310 nm 1-8 Gly 48 4-8 1-50 at 320 nm 3.1 Gly 72 4.7 1.46 at 310 nm 3-2 Lys 24 26.2 0.9 at 330 nm 7-2 Ala 24 0 Set 24 0 Arg 24 0 Underlined wavelengths correspond to a maximum. The yield in melanoidin obtained for lysine as well as its free spin content, show by comparison with the results obtained at 100øC, that the reaction is already in an advanced stage after 24 h at 37øC. For glycine however, 48 h are necessary to obtain the same num- ber of free spins/g, and after 72 h the pigment yield is still much lower than at higher temperature. But in spite of these quantitative differences, the DHA melanoidins prepared at 37 ¸ or 100øC are very similar as far as their U.V., I.R. and E.S.R. spectra are concerned. Discussion Comparison with melanins One of the most important characteristics of melanins, the natural pigments of hair, skin, squid ink... is to give an E.S.R. signal of 4.8 (6) to 8.8 G (7) linewidth depending upon the origin of the sample and perhaps also upon the conditions used in recording the spectra. It corresponds to free spin contents ranging from 5 x 10XS/g (6, 8) to 20 x 10XS/g (9) for Sepia melanin, and from 0.4 x 10•8/g (8) to 2 x 10X*/g (9) for synthetic melanin prepared by oxidation of DOPA. These data are strikingly similar to those of Table IV. Moreover, U.V. visible absorption spectra of melanins which have no special features
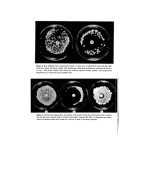
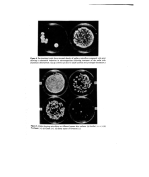
32 A. Meybeck (6) or just a maximum at 327 nm (8), also resemble those of melanoidins. And as far as I.R. is concerned the same bands are found again for both types of pigments since melanins show, as melanoidins do (Table III), a maximum around 3400, and a maximum around 1620 with a shoulder near 1700 cm -x (6, 7, 10, 11). Their percentage compositions are a little different, however, since a typical hair melanin contains 57'1•o C, 3.55/o H, 9'6•o N and 29'4•o O (11) while we found for the (DHA+Gly) melanoidin: 46.75/o C, 5'55/o H and 8-45/o N, the remaining unaccounted for 39'4•o being probably oxygen. All this shows that, although the pigments formed in the reaction of DHA with aminoacids are different by their preparation, they are nevertheless very similar to melanins by their properties and especially by their free spin content. Hypotheses on the structure of melanoidins Still another very interesting result is that E.S.R. spectra of melanoidins can be observed in their aqueous reaction media at room temperature (Fig. 4) showing that the free spin centres, if they were real free radicals, ought to be extremely inert. And it is particularly striking to remark that an unpurified solution of (DHA +Gly) pigment prepared at 37øC and never heated above this temperature, also exhibits a signal. The E.S.R. absorption lines observed in water have the same linewidths (7'5-9.5 G) and apparently the same intensity as the dry powders, although this last point is difficult to certify since a special flat cell of unknown volume had to be used in these experiments due to the high dielectric loss of aqueous solutions. These last results particularly, show that the E.S.R. behaviour of melanoidins is an intrinsic property of their macromolecule and is not due to some kind of free radicals trapped during polymerization. Io G Gly Solutions / • GI Copsule Dial. Und•ol, Figure 4. E.S.R. spectra of: solutions of melanoidins obtained by reacting DHA with glycine for 24 h at 37øC and 6 h at 100øC natural dispersions of melanin pigments in light and dark hair malanoidin obtained by action of DHA on glycine in an open capsule over a steam bath until the reaction medium was dry, and observed in the powder state before and after purification by dialysis. In the following experiment, however, such species may have been formed. DHA (1'8 g) and Gly (1.5 g) were heated with 6 g water in a capsule on a steambath until the brown resultant mass was dry (1 h 30 min). A sample of this unpurified product was kept and
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)