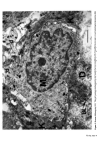
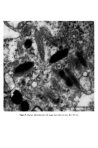
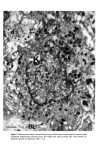
Causes of skin colouration 625 He felt that cross-linking of these filaments both at the periphery and at the centre of the melanosome was responsible for the transverse striations seen in micrographs. After formation of the protein matrix, melanin deposition gradually occurs, and the pigment accumulates on the inner membranes obscuring the characteristic periodicity of the structure. Finally the organelle becomes a uniformly dense particle without discernible internal structure. Four stages in the development of the melanosome are recognized (13): Stage I. A spherical, membrane delineated vesicle may be called a melanosome if it: (i) is shown to contain tyrosinase by electron microscopy combined with histo- chemistry or (ii) contains filaments that have a distinct periodicity of 100A. Stage II. The organelle is oval and shows numerous membranous filaments, with or without cross linking, having a distinct periodicity. Stage III. The internal structure, characteristic of Stage II has become partially obscured by electron-dense melanin. Stage IV. The oval organelle is electron-opaque without discernible internal structure in routine preparations. Stage I melanosomes are seen as spherical vesicles near the Golgi apparatus. The other stages are usually seen scattered singly throughout the cytoplasm (Fig. 5) though there is a preponderance of Stage III and Stage IV melanosomes in the dendritic pro- cesses. If preservation is good a distinct unit membrane can be seen surrounding the internal structure of the organelle (Fig. 4). Occasionally complexed melanosomes are also seen in normal melanocytes. Intracellular site of melanin synthesis Information obtained from electron microscopy, electron microscopic cytochemistry, autoradiography and cell particle fractionation supports the view that tyrosinase is synthesised on the ribsosomes. It is then transferred via the rough endoplasmic reticu- lum to the Golgi apparatus from where it is channelled via tubular elements to a focal dilatation of the smooth endoplasmic reticulum in which the coiled melanosomal matrix has independently formed. Melanisation of the structural protein can then take place and once this is completed the connection with tubular system is severed (14, 15, 16). RACIAL DIFFERENCES IN PIGMENTATION It seems extraordinary that it was not until the late nineteen sixties that Man began to understand why Negroes were black and Caucasoids white. The work of Szabo, Wolff and their colleagues (17, 18, 19) has clarified the problem and is worth summarising. There is no difference in the number of melanocytes between Negroes and Caucasoids (20). There are, however, fewer melanosomes in the melanocytes and keratinocytes of Caucasoids and Mongoloids. Of those present in the melanocyte most are in Stages I, II and III. Those in the keratinocytes are in Stage IV but tend to be grouped in membrane- limited organelles to form 'melanosome complexes'. The appearance is different in Negroids and Australian aborigines. Here there are more melanosomes in the melanocytes and keratinocytes and a high proportion of melanosomes are seen at the IVth stage of
626 J. A. A. Hunter development. Most of the melanosomes in keratinocytes appear disposed individually rather than in complexes (Fig. 5) (17). Wolff and Konrad (18, 19) have shown, both experimentally (using latex beads in guinea pig skin) and in human pigmentary dis- orders, that the complexing of melanosomes in keratinocytes is a size-dependent pheno- menon. Particles of 0.1 I• tended to be complexcd whilst those with a diameter of 0.8 I•m were not. CAUCASOlD KERA]'INOCY]'E NEGROID KERATINOCYTE Figure 8. Racial differences in melanosomal packaging. The smaller melanosomes in the Caucasoid keratinocyte are usually complexed whereas the larger ones in the Negroid tend to be disposed individually. REFERENCES 1 Findlay, G. H. Blue Skin. Brit. J. Derrn. 83 127 (1970). 2 Bloch, B. Chemische Untersuchungen fiber das spezifische pigmentbildende Ferment der Haut, die Dopa oxidase. Zeitschr. f. physiol. Chern. xcviii 226 (1917). 3 Becker, S. W. Praver, L. L. and Thatcher, H. An improved (paraffin section) method for the Dopa reaction. With considerations of the dopa-positive cell, as studied by this method. Arch. Derrn. Syoeh. 31 190 (1935). 4 Masson, P. Pigment cells in Man. In: The Biology of Melanomas. IV. Miner, R. W. and Gordon, M., Eds, Special publication, New York Academy of Sciences, 1948, p. 15. 5 Fitzpatrick, T. B., Becker, S. W. Jr., Lerner, A. B. and Montgomery, H. Tyrosinase in Human Skin: Demonstration of its presence and of its Role in Human Melanin Formation. Science, 112 223 (1950). 6 Billingham, R. E. Dendritic Cells. J. Artat. 82 93 (1948). 7 Cruickshank, C. N. D. and Harcourt, S. A. Pigment Donation in vitro. J. invest. Derrn. 42 183 (1964). 8 Fitzpatrick, T. B. and Breathnach, A. S. Das epidermale Melanin-Einheit-system. Derrn. Wschr. 147 481 (1963). 9 Rawles, M. E. Origin of melanophores and their r61e in development of color patterns in vertebrates. Physiol. Rev. 28 383 (1948). 10 Breathnach, A. S. and Wyllie, L. M. Electron Microscopy of Melanocytes and Langerhans cells in Human Fetal Epidermis at fourteen weeks. J. Invest. Derrn. 44 51 (1965). 11 Duchon, J., Fitzpatrick, T. B. and Seiji, M. Melanin some definitions and problems. In: Year Book ofDerrnatology. Kopf, A. W. and Andrade, a d R., Eds, Year Book Medical Publishers, Chicago, 1968, p. 6. 12 Drochmans, P. Ultrastructure of melanin granules. In: Advances in Biology of Skin: The Pig- rnentary System. VIII. Montagna, W. and Hu, F., Eds, Pergamon Press, Oxford, 1967, p. 169,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)