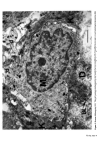
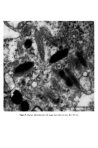
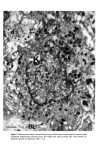
Causes of skin colouration 627 13 Fitzpatrick, T. B., Quevedo, W. C. Jr., Szabo, G. and Seiji, M. Biology of the melanin pigmentary system. In: Dermatology in General Medicine. Fitzpatrick, T. B., Arndt, K. A., Clark, W. H., Eison, A. Z., 'Van Scott, E. J. and Vaughan, J. H., Eds, McGraw Hill, New York, 1971, p. 117. 14 Seiji, M., Fitzpatrick, T. B. and Birbeck, M. S.C. The Melanosome: a distinctive subcellular particle of mammalian melanocytes and the site of melanogenesis. J. invest. Derre. 36 243 (1961). 15 Maul, G. G. Golgi-melanosome relationship in human melanoma in vitro. J. ultrastruct. Res. 26 163 (1969). 16 Hunter, J. A. A., Mottaz, J. H. and Zelickson, A. S. Melanogenesis: ultrastructural histochemical observations on ultraviolet irradiated human melanocytes. J. invest. Derrn. 54 213 (1970). 17 Szabo, G., Gerald, A. B., Pathak, M. A. and Fitzpatrick, T. B. Racial differences in the fate of melanosomes in human epidermis. Nature, 222 (1969). 18 Wolff, K. and Konrad, K. Phagocytosis of latex beads by epidermal keratinocytes in vivo. J. ultrastruct. Res. 39 262 (1972). 19 Konrad, K. and Wolff, K. Hyperpigmentation, melanosome size, and distribution patterns of melanosomes. Arch. Derre. 107 853 (1973). 20 Billingham, R. E. Dendritic cells in pigmented human skin. J. Anat. 83, 109 (1949).
J. Soc. Cosmet. Chem. 28 629-639 (1977) ¸ 1977 Society of Cosmetic Chemists of Great Britain Alkyl substituted 5.methylcyclohex-2-en-l-ones B R U C E A. McAN D RE W Research Laboratories, Proprietary Perfumes Limited, Ashford, Kent Presented at the Joint Symposium with the British Society of Perfumers, "The future of perfumery" 4 April 1977, Stratford-upon-A yon. Synopsis The versatility of Hagemann's Ester (ethyl 2-methyl-4-oxocyclohex-2-ene-l-carboxylate) as an inter- mediate in the synthesis of a range of alkylated 3-methylcyclohex-2-en-l-ones has been demonstrated. The effects of the substitution pattern and of the chain length of the alkyl substituent on the odour of these ketones are discussed and some comparisons drawn with their cyclopentenone analogues (dihydrojasmones). The correlation of structure with odour is a continuing challenge to the perfumery chemist. It is one which has stimulated considerable research over the last 20 years and one to which there has been, as yet, no definitive answer. Dihydrojasmone [1] and Lyral [3] are chemicals extensively used in our industry. When these compounds are drawn in the orientation shown, it can be readily appreciated that a 2-alkyl-methylcyclohex-2-en-l-one [e.g. 2] exhibits structural features common to both, and consequently could well possess an interesting odour. i 2 5 The preparation of these materials utilised Hagemann's Ester [7] as the starting material. This compound was prepared by the base catalysed condensation of formal- dehyde [5] with ethyl acetoacetate [4] in a molar ratio of 1:2 this produced the diester [6] as the first isolable intermediate (1). 629
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)